- 0086-21-56469616
- 0086-18019205509
- minstar@minstargroup.com
- Language:English
- English
Your Location:Home >Products >Pharmaceutical >1257044-40-8
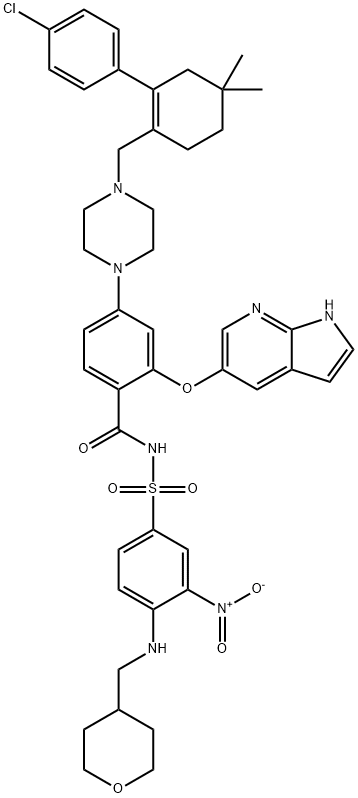

Purity:99%
|
1257044-40-8 Name |
|
|
Name |
ABT-199 |
|
Synonym |
ABT-199;ABT-199 (GDC-0199);GDC-0199;ABT-199 100MG;2-(1H-Pyrrolo[2,3-b]pyridin-5-yloxy)-4-(4-((2-(4-chlorophenyl)-4,4-dimethylcyclohex-1-enyl)met;ABT-199, Venetoclax;ABT-199 (GDC-0199)Venetoclax;4-[4-[[2-(4-Chlorophenyl)-4,4-dimethyl-1-cyclohexen-1-yl]methyl]-1-piperazinyl]-N-[[3-nitro-4-[[(tetrahydro-2H-pyran-4-yl)methyl]ami;Venclexta |
|
1257044-40-8 Chemical & Physical Properties |
|
|
Density |
1.3±0.1 g/cm3 |
|
Molecular Formula |
C45H50ClN7O7S |
|
Molecular Weight |
868.439 |
|
PSA |
186.58000 |
|
LogP |
10.88 |
|
Exact Mass |
867.318115 |
|
Index of Refraction |
1.644 |
|
Water Solubility |
Insuluble (6.3E-6 g/L) (25 ºC) |
ABT-199, also known as venetoclax, is a small, oral chemical molecule used for the treatment of chronic lymphocytic leukemia (CLL) associated with a specific chromosomal abnormality. As a second line treatment drug of CLL, ABT-199 take effects through acting as a BH3-mimetic and inhibitor of Bcl-2 (the anti-apoptotic B-cell lymphoma-2protein), further inducing apoptosis of CLL cells but not platelets. It also has the potential for the treatment of ER-positive breast cancer. As a specific Bcl-2 inhibitor of Bcl-2, ABT-199 was approved by FDA to treat CLL in 2015.
The present invention relates to a process for the preparation of 4-(4-{[2-(4-chlorophenyl)-4,4dimethylcyclohex-1-en-1-yl]methyl}piperazin-1-yl)-N-({3-nitro-4-[(tetrahydro-2H-pyran-4ylmethyl)amino]phenyl}sulfonyl)-2-(1H-pyrrolo[2,3-b] pyridin-5-yloxy)benzamide) compound of formula-1 which is represented by the following structural formula: Formula-1.
The combination of ABT-199 with JAK1/2 inhibitor Ruxolitinib or STAT3 inhibitors Stattic and C188-9 increased malignant B cell death, thus blocking this pathway with inhibitors increased ABT-199 efficiency to induce CLL cell apoptosis, suggesting a potential therapeutically relevant combination to overcome ABT-199 resistance.
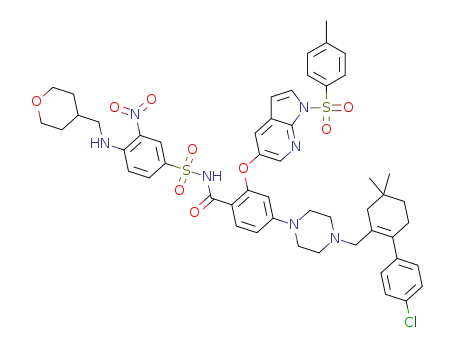
C52H56ClN7O9S2

![2-((1H-pyrrolo[2,3-b]pyridin-5-yl)oxy)-4-(4-((4'-chloro-5,5-dimethyl-3,4,5,6-tetrahydro-[1,1-biphenyl]-2-yl)methyl)piperazin-1-yl)-N-((3-nitro-4-(((tetrahydro-2H-pyran-4-yl)methyl)amino)phenyl)sulfonyl)benzamide](/upload/2023/1/8d0b2511-7e44-4a1d-8585-698fbb18e602.png)
2-((1H-pyrrolo[2,3-b]pyridin-5-yl)oxy)-4-(4-((4'-chloro-5,5-dimethyl-3,4,5,6-tetrahydro-[1,1-biphenyl]-2-yl)methyl)piperazin-1-yl)-N-((3-nitro-4-(((tetrahydro-2H-pyran-4-yl)methyl)amino)phenyl)sulfonyl)benzamide
| Conditions | Yield |
|---|---|
|
With lithium hydroxide; In methanol; at 20 - 68 ℃;
|
90% |
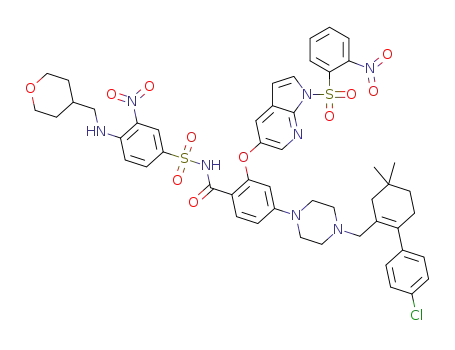
C51H53ClN8O11S2

![2-((1H-pyrrolo[2,3-b]pyridin-5-yl)oxy)-4-(4-((4'-chloro-5,5-dimethyl-3,4,5,6-tetrahydro-[1,1-biphenyl]-2-yl)methyl)piperazin-1-yl)-N-((3-nitro-4-(((tetrahydro-2H-pyran-4-yl)methyl)amino)phenyl)sulfonyl)benzamide](/upload/2023/1/8d0b2511-7e44-4a1d-8585-698fbb18e602.png)
2-((1H-pyrrolo[2,3-b]pyridin-5-yl)oxy)-4-(4-((4'-chloro-5,5-dimethyl-3,4,5,6-tetrahydro-[1,1-biphenyl]-2-yl)methyl)piperazin-1-yl)-N-((3-nitro-4-(((tetrahydro-2H-pyran-4-yl)methyl)amino)phenyl)sulfonyl)benzamide
| Conditions | Yield |
|---|---|
|
With caesium carbonate; In isopropyl alcohol; at 20 - 80 ℃;
|
92% |
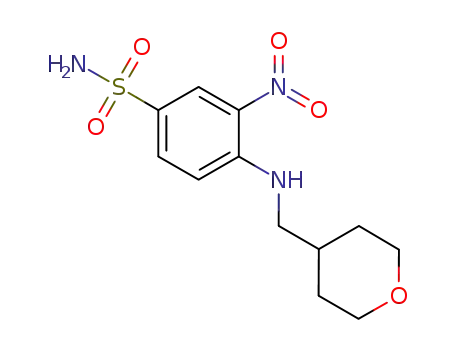
3-nitro-4-(((tetrahydro-2H-pyran-4-yl)methyl)amino)benzenesulfonamide
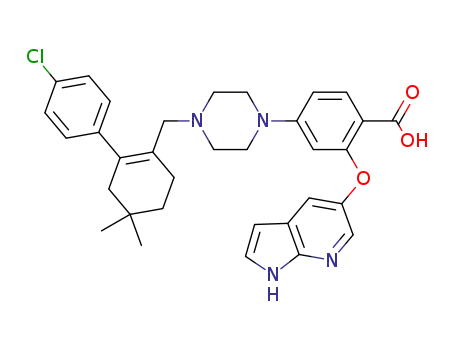
[2-((1H-pyrrolo [2,3-b]pyridin-5-yl)oxy)-4-(4-((4’-chloro-5,5-dimethyl-3,4,5,6-tetrahydro-[1,1’-biphenyl]-2-yl)methyl)piperazin-1-yl)benzoic acid]
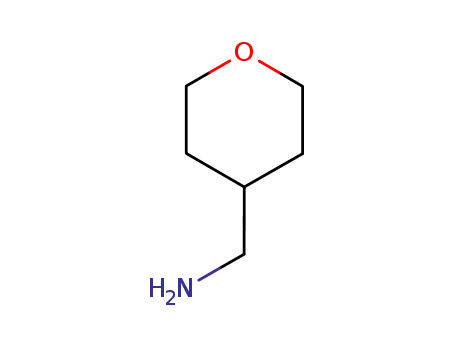
4-tetrahydropyranmethylamine
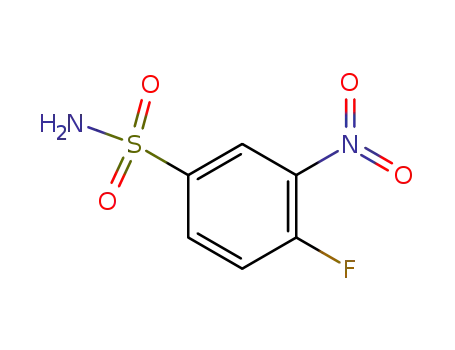
4-fluoro-3-nitrobenzenesulfonamide
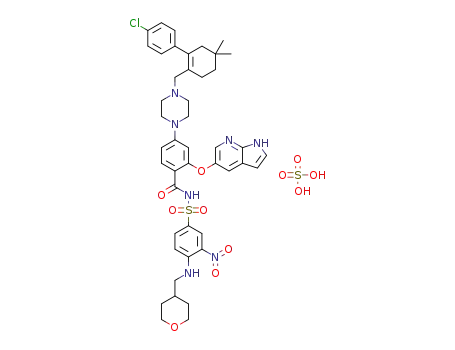
4-(4-{[2-(4-chlorophenyl)-4,4-dimethylcyclohex-1-en-1-yl]methyl}piperazin-1-yl)-N-({3-nitro-4-[(tetrahydro-2H-pyran-4-ylmethyl)amino]phenyl}sulfonyl)-2-(1H-pyrrolo[2,3-b]pyridin-5-yloxy)benzamide sulfate
CAS:127426-30-6
CAS:89466-55-7
CAS:987-65-5
CAS:112926-00-8