- 0086-21-56469616
- 0086-18019205509
- minstar@minstargroup.com
- Language:English
- English
Your Location:Home >Products >Pharmaceutical >378-44-9

Appearance:white to off-white solid
Purity:99%
|
378-44-9 Name |
|
|
Name |
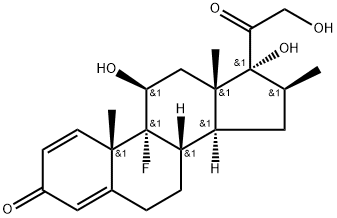
Betamethasone |
|
Synonym |
17,21-trihydr;betamethasone standard;(8S,9R,10S,11S,13S,14S,16S,17R)-9-fluoro- 11,17-dihydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl- 6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3H-cyclopenta[a]phenanthren-3-one;Betamethasone Base & Salts;Betamethasone,9α-Fluoro-11β,17α,21-trihydroxy-16β-methylpregna-1,4-diene-3,20-dione, 9α-Fluoro-16β-methyl-11β,17α,21-trihydroxy-1,4-pregnadiene-3,20-dione, 9α-Fluoro-16β-methylprednisolone;Betamethasone (200 mg);BetaMethasone (Celestone);BetaMethasone SolutioM |
|
378-44-9 Biological Activity |
|
|
Related Catalog |
Signaling Pathways >> GPCR/G Protein >> Glucocorticoid Receptor Research Areas >> Inflammation/Immunology |
|
378-44-9 Chemical & Physical Properties |
|
|
Melting point |
235-237°C |
|
Boiling point |
568.2±50.0 °C at 760 mmHg |
|
Density |
1.3±0.1 g/cm3 |
|
Molecular Formula |
C22H29FO5 |
|
Molecular Weight |
392.461 |
|
Flash Point |
297.5±30.1 °C |
|
PSA |
94.83000 |
|
LogP |
1.87 |
|
Exact Mass |
392.199890 |
|
Vapour Pressure |
0.0±3.5 mmHg at 25°C |
|
Index of Refraction |
1.592 |
Betamethasone, a corticosteroid drug used in the clinic for its anti-inflammatory and immunosuppressive effects, were effective in reducing the intensity of epileptic seizures. Betamethasone (BM) is the drug of choice for antenatal corticosteroid therapy for women at risk of preterm delivery because it induces fetal lung maturation and enhances survival after birth.
InChI:InChI=1/C22H29FO5/c1-12-8-16-15-5-4-13-9-14(25)6-7-19(13,2)21(15,23)17(26)10-20(16,3)22(12,28)18(27)11-24/h6-7,9,12,15-17,24,26,28H,4-5,8,10-11H2,1-3H3/t12-,15+,16+,17+,19+,20+,21+,22+/m1/s1
A variation of the Mattox rearrangement, a key degradation pathway under acidic conditions for corticosteroids possessing the 1,3-dihydroxyacetone side chain, has been found to occur for the 17,21-diesters of these corticosteroids but under the alkaline condition. The mechanism of this variation of the original Mattox rearrangement is proposed.
To review the pharmacokinetics, efficacy, and safety of recently approved calcipotriene and betamethasone dipropionate (C-BD) cream. Newly FDA-approved C-BD cream with novel polyaphron dispersion (PAD) technology provides a safe efficacious combination therapy for mild-to-moderate psoriasis which may be preferred by some patients.
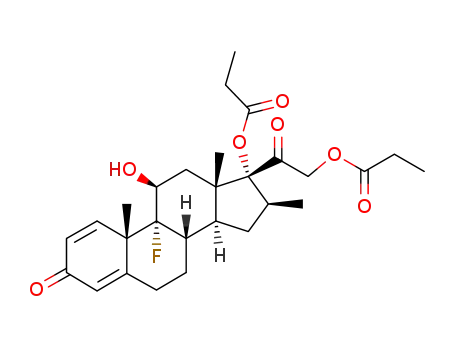
betamethasone dipropionate

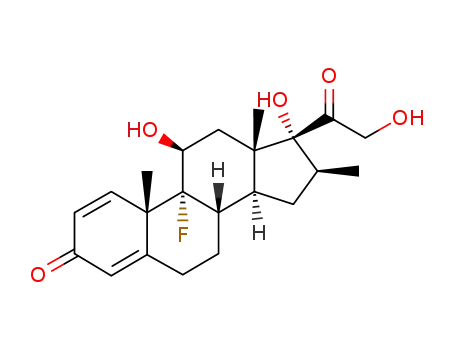
betamethasone

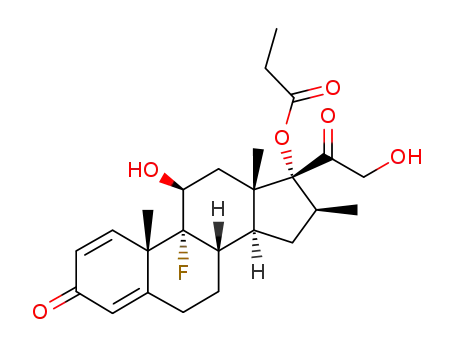
Betamethasone propionate

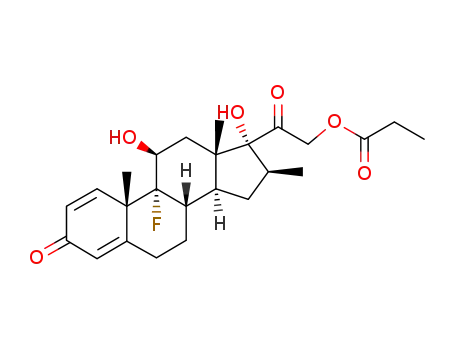
betamethasone 21-monopropionate
| Conditions | Yield |
|---|---|
|
With sulfuric acid; In acetonitrile; at 20 ℃; for 20h; Further Variations:; Temperatures; Product distribution;
|

betamethasone dipropionate


betamethasone


Betamethasone propionate

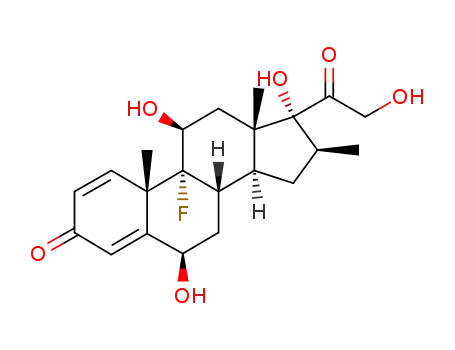
6β-hydroxybetamethasone

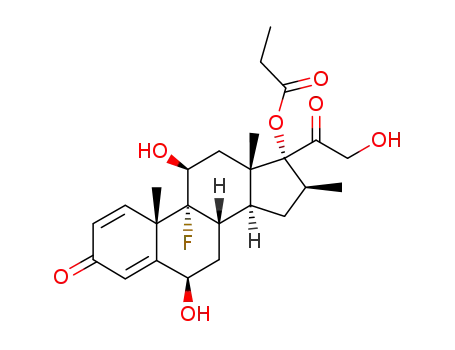
6β-hydroxybetamethasone 17-propionate
| Conditions | Yield |
|---|---|
|
With phosphate buffer; air; plasma of 20 d pregnant Sprague-Dawley rat; at 37 ℃; for 1h; Product distribution; metabolism with tissues (plasma, liver, brain, placenta) from mothers and fetuses of Sprague-Dawley rats sacrificed on day 20 of pregnancy and mice on day 17 of pregnancy, further in vivo;
|
67.8 % Chromat. 2.3 % Chromat. 14.8 % Chromat. 1.4 % Chromat. |

betamethasone dipropionate
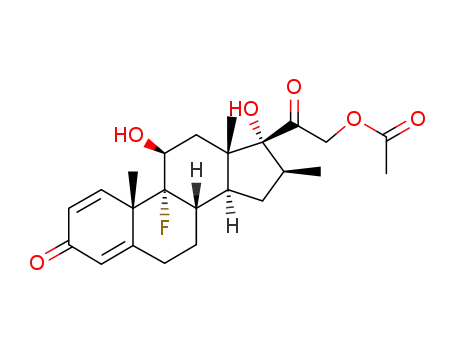
betamethasone 21-acetate
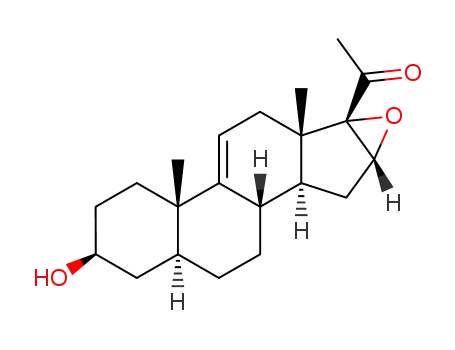
16α,17α-epoxy-3β-hydroxy-5α-pregn-9(11)-en-20-one
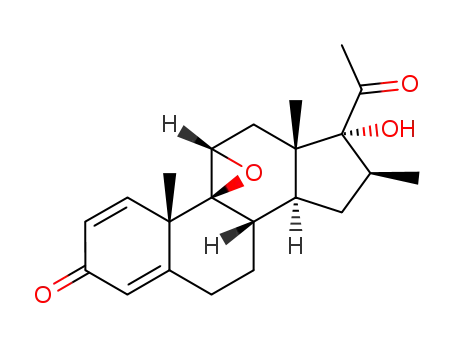
16β-methyl-9β,11β-epoxy-17α-hydroxy-1,4-pregnadiene-3,20-dione
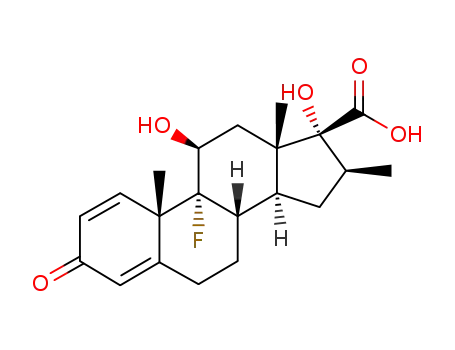
9α-fluoro-11β,17α-dihydroxy-16β-methyl-3-oxo-androsta-1,4-diene-17β-carboxylic acid

Betamethasone propionate
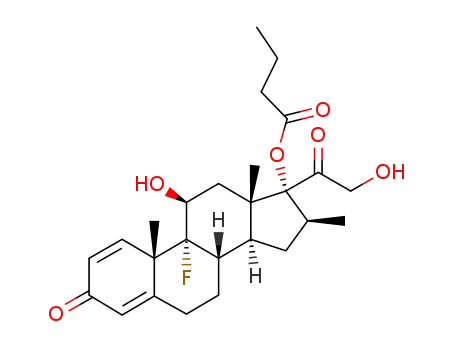
9α-fluoro-11β,17α,21-trihydroxy-16β-methyl-1,4-pregnadiene-3,20-dione 17-butyrate
betamethasone-valerate
CAS:517920-73-9
CAS:881681-00-1
CAS:87-89-8
CAS:53014-84-9