- 0086-21-56469616
- 0086-18019205509
- minstar@minstargroup.com
- Language:English
- English
Your Location:Home >Products >40273-45-8
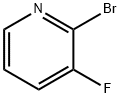

Appearance:colorless to slightly yellow liquid
Purity:99%
Chemical Properties
Colorless to slightly yellow liquid
A series of pyridinyltetrahydropyridine derivatives was synthesized and evaluated as adrenoceptor and tetrabenazine antagonists. 4-(3-Fluoro-2-pyridinyl)-1,2,5,6-tetrahydropyridine proved to be the most potent and selective α2-adrenoceptor antagonist of the series as measured in vitro by displacement of 3H>clonidine and 3H>prazosin from membrane binding sites of calf cerebral cortex and by antagonism of the effects of clonidine and methoxamine in the rat isolated, field-stimulated vas deferens.In addition, this compound, and the corresponding desfluoro derivative, blocked tetrabenazine-induced ptosis in the mouse.
Metallation of ?-deficient heterocyclic compounds is first reviewed, which shows the important recent developments in this research area.A particular aspect of this reaction is then given with the study of the 3-fluoropyridine metallation regioselectivity.Lithiation of 3-fluoropyridine is chemoselective at low temperatures using butyllithium-polyamine chelates or lithium diisopropylamide.Protophilic attack by these strong bases can be directed either at the 2- or 4-position depending on the lithiation conditions.Various reaction parameters are thus studied such as solvent, temperature, reaction time, lithium-chelating agent as well as metallating agent.The high regioselectivity of 3-fluoropyridine lithiation is theoretically discused, in particular in terms of kinetic of thermodynamic control of the metallation.Chelation between butyllithium and 3-fluoropyridine is proposed, which completely modifies the heterocycle reactivity toward the lithiating agent.This is confirmed by theoretical quantum calculations performed on different models of 3-fluoropyridine using the CNDO/2.These results allow to select the best 3-fluoropyridine-metallation conditions which lead to 3-fluoro-2-lithiopyridine on the one hand and to 3-fluoro-4-lithiopyridine on the other hand.Each of the lithiated isomers is then reacted with a great variety of electrophiles which gives very conveniently the corresponding 2,3- or 3,4-disubstituted pyridines.
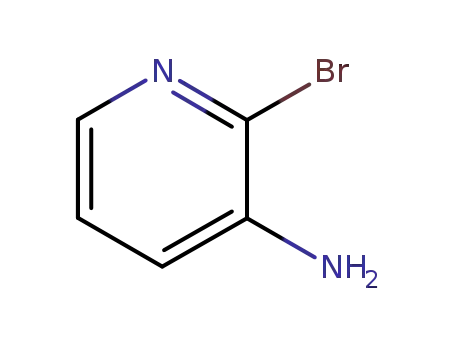
3-amino-2-bromopyridine

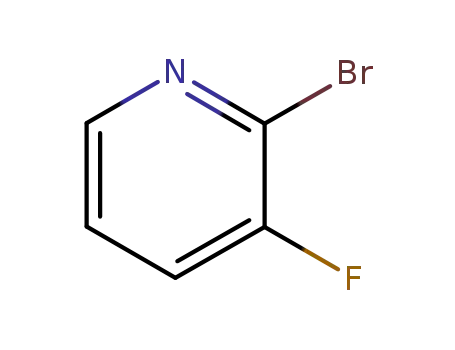
2-bromo-3-fluoropyridine
| Conditions | Yield |
|---|---|
|
With tetrafluoroboric acid; ethyl nitrite; Yield given. Multistep reaction; 1) ethanol, 0 deg C; 2) benzene, rt;
|
|
|
With hydrogenchloride; hexafluorophosphoric acid; sodium nitrite; Yield given. Multistep reaction; 1.) a) from -5 deg C to 0 deg C, 30 min, b) 0 deg C, 1 h, 2.) mineral oil, from 90 deg C to 100 deg C;
|
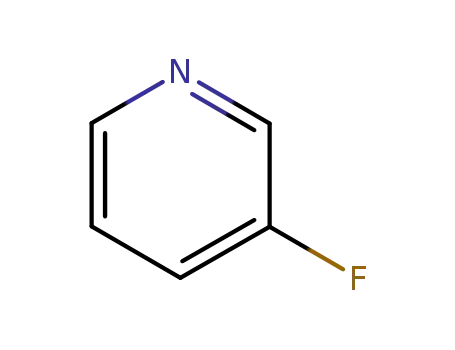
3-Fluoropyridine


2-bromo-3-fluoropyridine
| Conditions | Yield |
|---|---|
|
With n-butyllithium; N,N,N,N,-tetramethylethylenediamine; bromine; Yield given. Multistep reaction; 1) diethyl ether, -75 deg C, 4 hr;
|

3-Fluoropyridine

3-amino-2-bromopyridine

3-Fluoropyridine
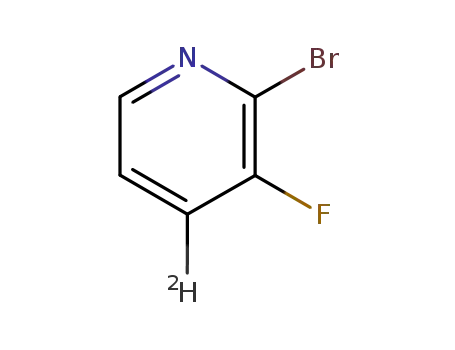
bromo-2 deuterio-4 fluoro-3 pyridine
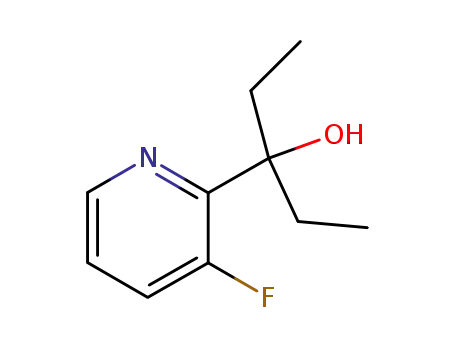
2-(1-hydroxy-1-ethylpropyl)-3-fluoropyridine
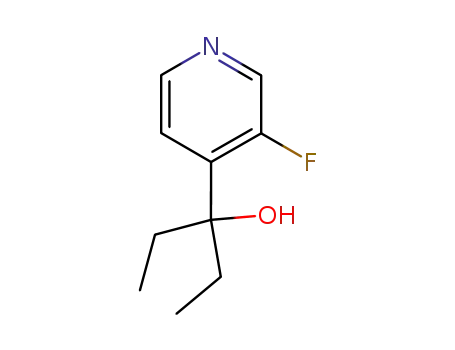
4-(1-hydroxy-1-ethylpropyl)-3-fluoropyridine
CAS:446-09-3
CAS:40273-45-8
CAS:31928-44-6
CAS:119515-38-7