Your Location:Home > Products > Pregabalin
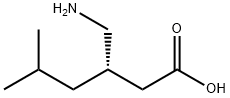

CasNo: 148553-50-8
Molecular Formula: C8H17NO2
Appearance: off-white solid
|
148553-50-8 Name |
|
|
Name |
Pregabalin |
|
Synonym |
3(S)-(AMINOMETHYL)-5-METHYLHEXANOIC ACID;(3S)-3-(AMINOMETHYL)-5-METHYLHEXANOIC ACID;PREGABALIN;Pregablin;3-(Aminomethyl)-5-methyl-hexanoic acid;PREDNISOLONESODIUMPHOSPHATE;(R)-Pregabalin;(S)-Pregabalin |
|
148553-50-8 Chemical & Physical Properties |
|
|
Melting point |
194-196ºC |
|
Boiling point |
274.0±23.0 °C at 760 mmHg |
|
Density |
1.0±0.1 g/cm3 |
|
Molecular Formula |
C8H17NO2 |
|
Molecular Weight |
159.226 |
|
Flash Point |
119.5±22.6 °C |
|
Exact Mass |
159.125931 |
|
PSA |
63.32000 |
|
LogP |
1.12 |
|
Index of Refraction |
1.465 |
|
Vapour Pressure |
0.0±1.2 mmHg at 25°C |
|
148553-50-8 Description |
|
Pregabalin is a second- generation antiepileptic drug (AED) known with the proprietary brand name of Lyrica® (Pfizer, Tadworth) in the UK and USA (Pfizer, New York, NY). Pregabalin (Lyrica), an analog of GABA that is related to gabapentin, has been extensively studied in the treatment of GAD, fibromyalgia, neuropathic pain, and partial complex seizures.It appears to act even more selectively on the a2-δ subunit of calcium channels found in brain than does gabapentin. Pregabalin received FDA approval in late 2004 for use in treating neuropathic pain and epilepsy. In 2008, it became the first drug approved for the treatment of fibromyalgia.Even though prcgabalin reccived approval for the treatment of GAD in the European Union in 2006, the FDA has not approvedpregabalin for GAD in the United States as of this writing.With the consistent efficacy in clinical trials and the known safety of pregabalin in clinical expericnce, it is not completely clear what has held up approval of prcgabalin for GAD.There was reported concern of the risk-benefit ratio for the drug,given the hepatotoxicity seen in micc, but this has not proven to be a problem postrelease in humans. Pregabalin is categorized as an anticonvulsant and analgesic. The drug is under re-review currently by the FDA. Pregabalin has a potential for abuse and misuse. Pregabalin is regulated as a Schedule V compound in the United States. |
|
148553-50-8 Uses |
|
Pregabalin has an approved indication and is widely used for the treatment of generalized anxiety disorder. Several randomized, double- blind, placebocontrolled trials found that pregabalin is an effective treatment for patients with generalized anxiety disorder and social anxiety disorder. Possible implications in the treatment of mood disorders and benzodiazepines dependence are emerging. Moreover, pregabalin may be a therapeutic agent for the treatment of alcohol abuse, in both withdrawal phase and relapse prevention. |