- 86-21-56469616
- 86-18019205509
- minstar@minstargroup.com
- Language:English
- English
Your Location:Home >Products >Fine Chemicals >14965-49-2
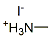
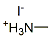
Appearance:powder
Throughput:100|Kilogram|Month
pd_productuse:intermediate
Delivery Time:in stock
Purity:98% purity
Melting point 270-280°C
Fp 12℃
form powder
Safety Information
Hazard Codes Xn
Risk Statements 22-36/37/38
Safety Statements 26-36/37/39-46-24/25
RIDADR UN1219 - class 3 - PG 2 - Isopropanol
WGK Germany 3
HS Code 29211100
MSDS Information
methylammonium iodide Usage And Synthesis
Specifications
Chemical formula CH6IN
Synonyms Methylamine hydroiodide
CAS No. 14965-49-2
Chemical name Methylammonium iodide
Physical appearance White, crystalline solid
Purification method Recrystallisation (ethanol)
Purity >99.9% (as measured by elemental analysis)
Molecular weight 158.97 g/mol
Recommended solvents for perovskite synthesis DMF, DMSO
Applications Methylammonium iodide (MAI), also referred to as methylamine hydroiodide, is a precursor for the synthesis of organic-inorganic hybrid perovskites for use in FETs, LEDs and PVs.
Due to the high purity of the methylammonium iodide (99.99%), it should be noted that its solubility is reduced within dimethyl formamide and dimethyl sulfoxide. This reduced solubility is due to the removal of trace amounts of residual hydroiodic acid (HI) used during the synthesis and purification of the material. This can potentially have an impact upon the performance of solar cells leading to a reduction in maximum power conversion efficiency achievable. Adding fixed concentrations of hydroiodic acid to perovskite solutions can allow for the improvement of device metrics. Using high-purity precursor materials allows for accurate addition of amounts of hydroiodic acid giving higher reproducibility to experiments. It is recommended that between 1% and 10% hydroiodic acid is used with high-purity methylammonium iodide to achieve optimal device performance. The amount required depends on the precursors used, solution concentration, solvent used, and processing environment. Therefore, this will need to be adjusted for each individual laboratory and process.
CAS:12008-78-5
CAS:
CAS:51309-10-5
CAS:403-14-5