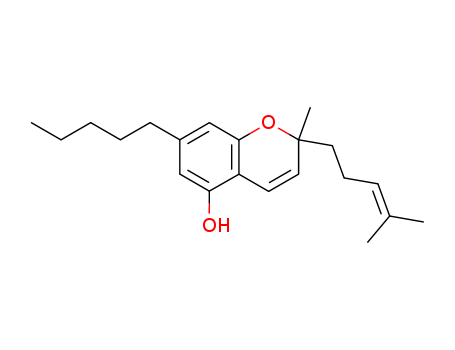

CasNo: 20675-51-8
Molecular Formula: C21H30 O2
|
20675-51-8 Name |
|
|
Name |
CBC |
|
Synonym |
Cannabichromone;Cannabichromene solution;Cannabichromene (CBC);2-methyl-2-(4-methylpent-3-enyl)-7-pentylchromen-5-ol;2-Methyl-2-(4-methyl-3-pentenyl)-7-pentyl-2H-1-benzopyran-5-ol;Cannabichrome;Pentylcannabichromene;cannabichromene |
|
20675-51-8 Chemical & Physical Properties |
|
|
Melting point |
145°C |
|
Boiling point |
428.7±45.0 °C at 760 mmHg |
|
Density |
1.0±0.1 g/cm3 |
|
Molecular Formula |
C21H30O2 |
|
Molecular Weight |
314.462 |
|
Flash Point |
174.2±23.0 °C |
|
PSA |
29.46000 |
|
LogP |
8.56 |
|
Exact Mass |
314.224579 |
|
Vapour Pressure |
0.0±1.1 mmHg at 25°C |
|
Index of Refraction |
1.527 |
|
Storage condition |
-20°C |
Cannabichromene (CBC), a new active principle in hashish, is one of four major cannabinoids in Cannabis sativa L. and is the second most abundant cannabinoid in drug‐type cannabis.
InChI:InChI=1/C21H30O2/c1-5-6-7-10-17-14-19(22)18-11-13-21(4,23-20(18)15-17)12-8-9-16(2)3/h9,11,13-15,22H,5-8,10,12H2,1-4H3
Cannabichromene (CBC, 1a) occurs in Cannabis (Cannabis sativa) as a scalemate having a composition that is strain-dependent in terms of both enantiomeric excess and enantiomeric dominance. Due to the powerful anticonvulsant activity of (racemic) synthetic CBC, this seems especially urgent for studies aimed at the management of intractable pediatric epilepsy.
That being said, there are still some cannabinoids that naturally occur as a pair of enantiomers, such as cannabichromene (CBC) and cannabicyclol (CBL). CBC had the best productivity by far of all four compounds used in this study. This was due in part to the very good solubility, but also a desirable loading.
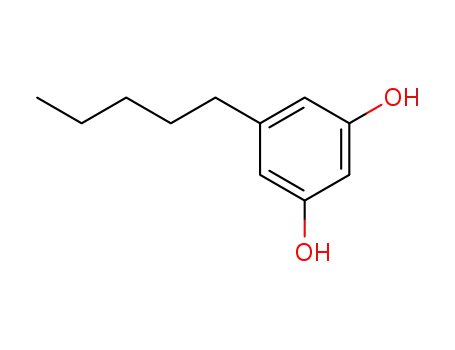
Olivetol

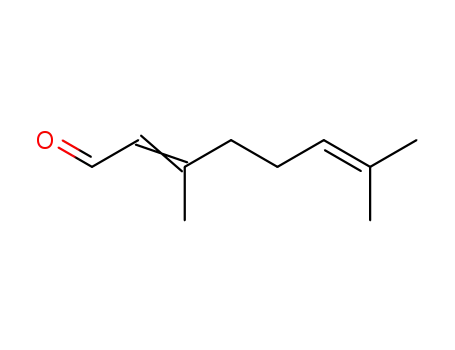
(E/Z)-3,7-dimethyl-2,6-octadienal

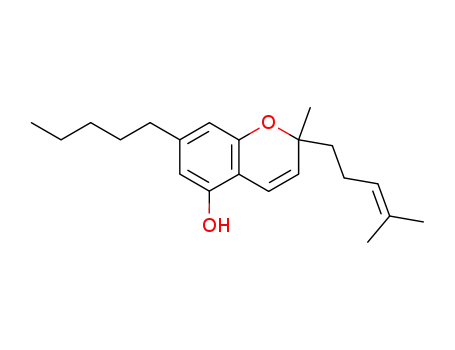
cannabichromene
| Conditions | Yield |
|---|---|
|
With ammonium chloride; In water; for 24h; Inert atmosphere; Reflux;
|
75% |
|
With tert-butylamine; In toluene; for 5h; Reflux; Dean-Stark;
|
74.5% |
|
With tert-butylamine; In toluene; for 18h; Time; Reagent/catalyst; Solvent; Inert atmosphere; Reflux;
|
61% |
|
(E/Z)-3,7-dimethyl-2,6-octadienal; With piperidine; acetic anhydride; In ethyl acetate; for 1h; Inert atmosphere; Sealed tube; Heating;
Olivetol; In ethyl acetate; toluene; at 130 ℃; for 40h; Inert atmosphere;
|
50% |
|
With acetic acid; ethylenediamine; In toluene; for 6h; Reflux;
|
38% |
|
With ethylenediamine diacetic acid; In toluene; at 110 ℃; for 6h; Inert atmosphere;
|
35% |
|
With propylamine; In toluene;
|
|
|
Olivetol; (E/Z)-3,7-dimethyl-2,6-octadienal; With N-butylamine; In toluene; Reflux;
In toluene; at 20 ℃; for 0.166667h;
|
|
|
(E/Z)-3,7-dimethyl-2,6-octadienal; With piperidine; acetic anhydride; In ethyl acetate; at 0 - 90 ℃; for 1h;
Olivetol; In ethyl acetate; toluene; at 139 ℃; for 65h; Inert atmosphere;
|
|
|
With tert-butylamine; In toluene; at 50 - 60 ℃; for 9h; Solvent; Temperature; Reflux;
|
|
|
Olivetol; With ethylenediamine; In toluene; at 100 ℃; Inert atmosphere; Reflux;
(E/Z)-3,7-dimethyl-2,6-octadienal; In toluene; at 100 ℃; Temperature; Inert atmosphere;
|

Olivetol


(E/Z)-3,7-dimethyl-2,6-octadienal


cannabichromene

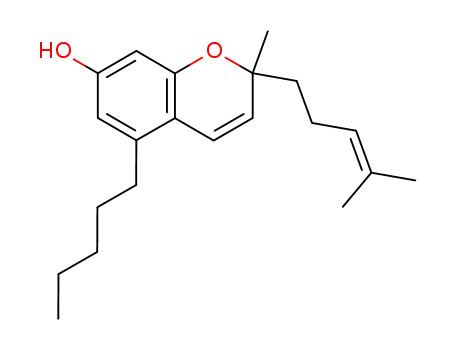
2-methyl-2-(4-methylpent-3-en-1-yl)-5-pentyl-2H-chromen-7-ol
| Conditions | Yield |
|---|---|
|
With triethylamine; In n-heptane; at 110 ℃; for 18h; Reagent/catalyst; Solvent; Temperature; Time; Inert atmosphere;
|
30% |
|
With Ti-doped montmorillonite; In toluene; at 80 ℃; for 2h; Schlenk technique;
|
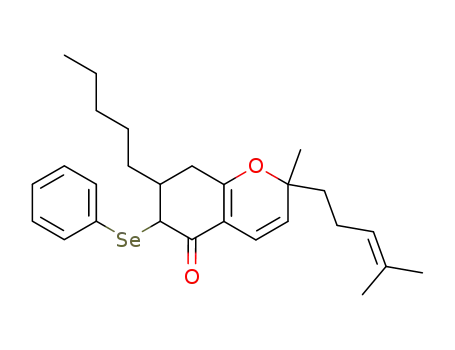
2-Methyl-2-(4-methyl-pent-3-enyl)-7-pentyl-6-phenylselanyl-2,6,7,8-tetrahydro-chromen-5-one
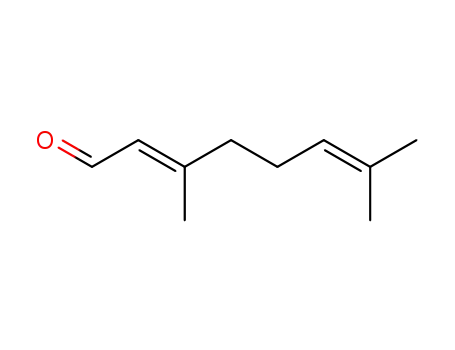
3,7-dimethyl-2,6-octadienal

Olivetol
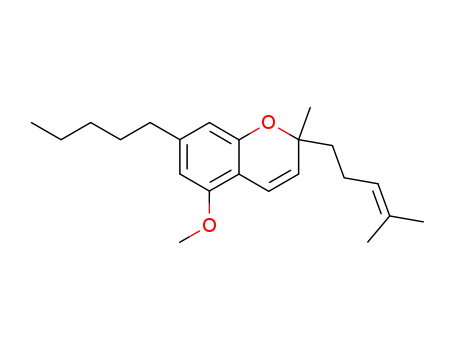
5-methoxy-2-methyl-2-(4-methyl-3-pentenyl)-7-pentyl-2H-chromene
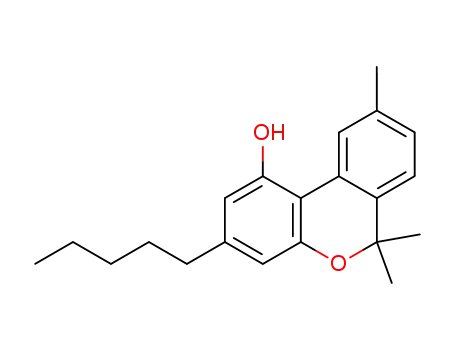
cannabinol
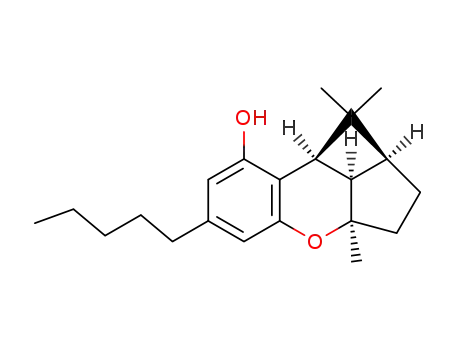
cannabicyclol
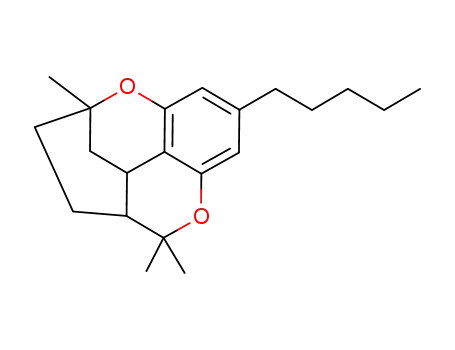
cannabicitran