Your Location:Home > Products > Fine Chemicals > 2,4,6-Collidine

CasNo: 108-75-8
Molecular Formula: C8H11N
Appearance: colourless liquid
|
108-75-8 Name |
|
|
Name |
2,4,6-Collidine |
|
Synonym |
2,4,6-Kollidin;2,4,6-trimethyl-pyridin;a,g,a'-Collidine;alpha,gamma,alpha’-collidine;alpha,gamma,alpha'-Collidine;g-Collidine;Pyridine,2,4,6-trimethyl-;2 Minus 2-3 Methyl pyridine |
|
108-75-8 Chemical & Physical Properties |
|
|
Melting point |
−43 °C(lit.) |
|
Boiling point |
171.0±9.0 °C at 760 mmHg |
|
Density |
0.9±0.1 g/cm3 |
|
Molecular Formula |
C8H11N |
|
Molecular Weight |
121.180 |
|
Flash Point |
57.2±0.0 °C |
|
PSA |
12.89000 |
|
LogP |
2.11 |
|
Exact Mass |
121.089149 |
|
Vapour Pressure |
1.9±0.3 mmHg at 25°C |
|
Index of Refraction |
1.502 |
|
Storage condition |
2-8°C |
|
Stability |
Stable. Combustible. Incompatible with strong oxidizing agents. |
|
Water Solubility |
35 g/L (20 ºC) |
2,4,6-Trimethylpyridine is a pyridine derivative. It has a pK of 7.4. The product can react with trifluoroiodomethane in cyclopentane solution to afford 1:1 complex. 2,4,6-collidine can used as monomer to produce a series of highly crystalline olefin-linked COFs by a melt polymerization method. This complex was investigated by NMR (Nuclear Magnetic Resonance) spectroscopy. 2,4,6-Collidine is used as a tissue fixative for electron microscopy. It is useful in dehydrohalogenation reactions and acts as a solvent for the cleavage of hindered esters by anhydrous lithium iodide. The crystal structure of 2,4,6-collidine (2,4,6-trimethylpyridine, C8H11N) has been determined at 180 (2) K following in situ crystal growth from the liquid.
Definition
Methyl, ethyl, propyl, and trimethyl homologs of pyridine.
InChI:InChI=1/C8H11N/c1-6-4-7(2)9-8(3)5-6/h4-5H,1-3H3
The association of pyridine and of 2,4,6-trimethylpyridine (collidine) with a pyramidal triarylborane belonging to the 9-boratriptycene family is investigated by NMR spectroscopy and by X-ray diffraction analysis. Despite of the large size of the ortho-disubstituted collidine Lewis base and of the large steric hindrance of the boron Lewis superacid used (9-boratriptycene-10-sulfonium), the superacidity at its boron atom is enabling the formation of a B-N bond and is resulting in a very stabe Lewis adduct.
In this report we describe further extension of the research on other derivatives of 3,5-diaryl substituted 2,4-, 2,6-lutidines and 2,4,6-collidines. In summary, we prepared a library of compounds using the Suzuki–Miyaura cross-coupling reaction between 3,5-dibromo-2,4,6-collidine and bromo derivatives of 2,6- and 2,4-lutidine with a series of ortho-substituted boronic acids.
2,4,6-Trimethylpyridiniumkation

naphthalene-1,4-diol; deprotonated form

2,4,6-trimethyl-pyridine

1,4-Dihydroxynaphthalene
| Conditions | Yield |
|---|---|
|
In acetonitrile; at 20 ℃; Equilibrium constant;
|
2,4,6-trimethylpyridinium picrate


2,4,6-trimethyl-pyridine

2,4,6-Trinitrophenol
| Conditions | Yield |
|---|---|
|
In acetic acid; at 25 ℃; Equilibrium constant;
|
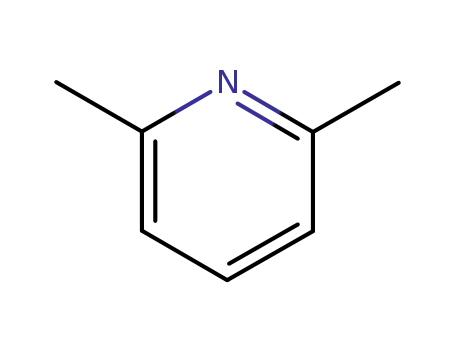
2,6-dimethylpyridine
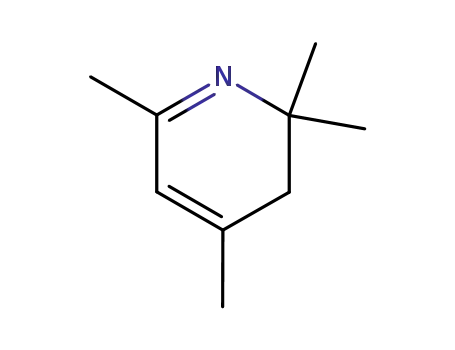
2,2,4,6-tetramethyl-2,3-dihydropyridine
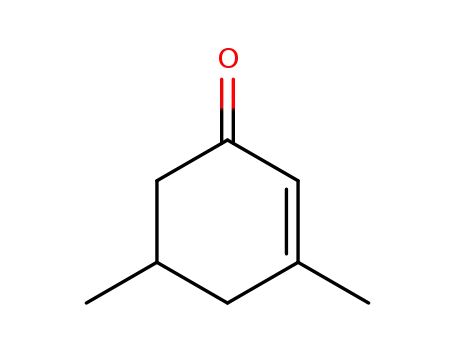
3,5-dimethyl-2-cyclohexen-1-one
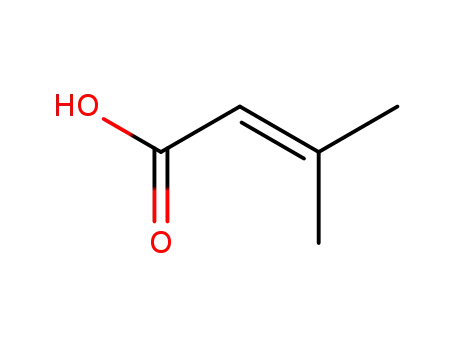
3-Methylbutenoic acid
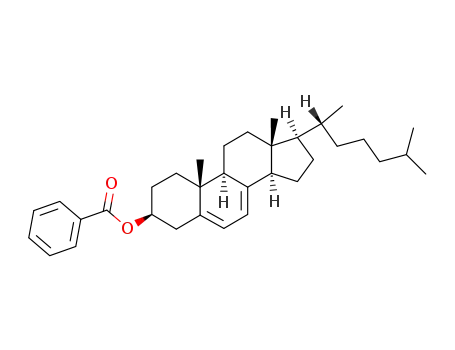
3β-benzoyloxycholesta-5,7-diene
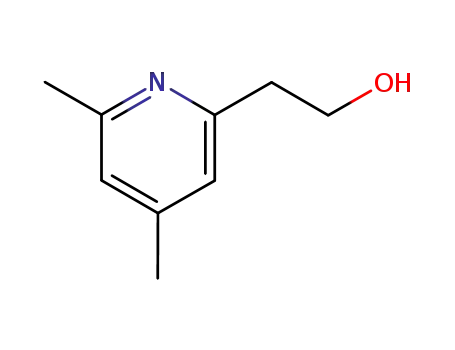
2-(4,6-dimethylpyridin-2-yl)ethanol
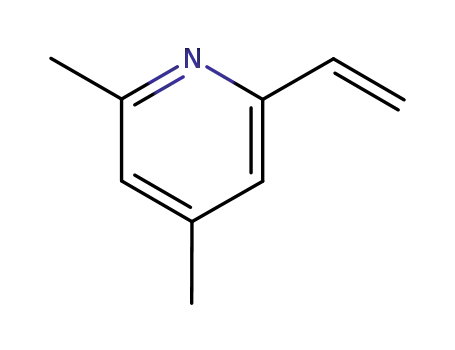
4,6-dimethyl-2-vinylpyridine
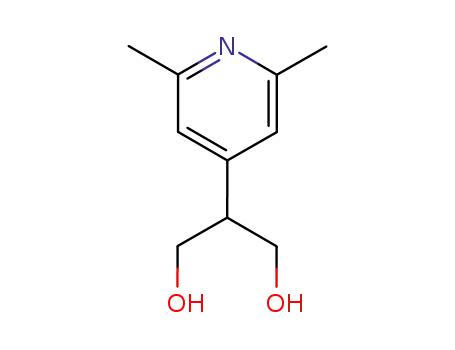
2-(2,6-dimethyl-[4]pyridyl)-propane-1,3-diol