Your Location:Home > Products > Fine Chemicals > 3-Pyridinecarboxaldehyde,6-methyl-
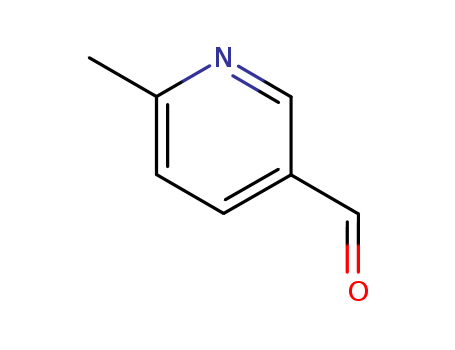

CasNo: 53014-84-9
Molecular Formula: C7H7NO
|
53014-84-9 Name |
|
|
Name |
3-FORMYL-6-METHYL-PYRIDINE |
|
Synonym |
6-METHYL-3-PYRIDINECARBOXALDEHYDE;5-FORMYL-2-PICOLINE;3-FORMYL-6-METHYL-PYRIDINE;6-Methylpyridine-3-carboxaldehyde;5-Formyl-2-methylpyridine;6-Methylnicotinaldehyde;3-Pyridinecarboxaldehyde, 6-methyl-;3-Formyl-6-methyl-pyridine ,97% |
|
53014-84-9 Chemical & Physical Properties |
|
|
Boiling point |
213.7±20.0 °C at 760 mmHg |
|
Density |
1.1±0.1 g/cm3 |
|
Molecular Formula |
C7H7NO |
|
Molecular Weight |
121.137 |
|
Flash Point |
87.6±29.2 °C |
|
PSA |
29.96000 |
|
LogP |
1.03 |
|
Exact Mass |
121.052765 |
|
Appearance of Characters |
Liquid |
|
Vapour Pressure |
0.2±0.4 mmHg at 25°C |
|
Index of Refraction |
1.563 |
InChI:InChI=1/C7H7NO/c1-6-2-3-7(5-9)4-8-6/h2-5H,1H3
The organomagnesium complex nBu2iPrMgLi, readily prepared from nBuLi and iPrMgCl (2:1), is quite efficient for the bromine-magnesium exchange of 5-bromo-2-picoline under noncryogenic conditions (at -10°C). The resulting picolylmagnesium complex reacts with various electrophiles to afford functionalized picolines.
Compounds of formula (VIII) and compositions for treating disorders related to TRPA1 are described herein.
Provided is a kynurenine production inhibitor comprising a nitrogen-containing heterocyclic compound represented by formula (I): (wherein R50 and R51 may be the same or different and each represent a hydrogen atom or the like, G1 and G2 may be the same or different and each represent a nitrogen atom or the like, X represents formula (III): (wherein m1 and m2 may be the same or different and each represent an integer of 0 or 1, Y represents an oxygen atom or the like, and R6 and R7 may be the same or different and each represent a hydrogen atom or the like), R1 represents optionally substituted lower alkyl or the like, R2 represents a hydrogen atom or the like, and R3 represents optionally substituted lower alkyl or the like), and the like.
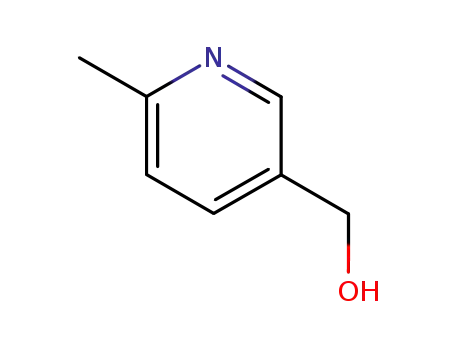
6-methyl-3-pyridinemethanol

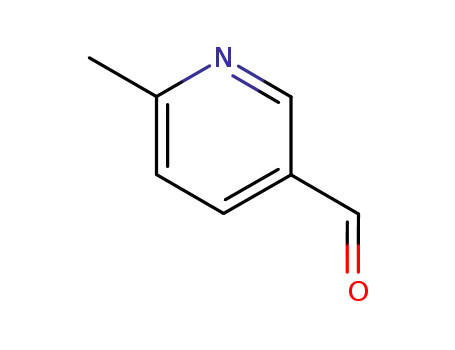
2-methyl-5-formylpyridine
| Conditions | Yield |
|---|---|
|
6-methyl-3-pyridinemethanol; With oxalyl dichloride; dimethyl sulfoxide; In dichloromethane; at -60 ℃; for 1.33333h;
With triethylamine; In dichloromethane; at -60 - 20 ℃; for 0.166667h;
|
85% |
|
With oxalyl dichloride; dimethyl sulfoxide; triethylamine; In dichloromethane; at -60 ℃; for 1.5h;
|
85% |
|
With lead(IV) acetate; In toluene; for 2.5h; Heating;
|
57% |
|
With oxalyl dichloride; dimethyl sulfoxide; triethylamine; Yield given. Multistep reaction; 1.) methylene chloride, -60 deg C, 20 min, 2.) from -60 deg C to RT;
|
|
|
In pyridine; water;
|
|
|
With manganese(IV) oxide; In dichloromethane; at 25 ℃; for 68h;
|
|
|
In pyridine; water;
|
|
|
With manganese(IV) oxide; In dichloromethane; at 0 - 20 ℃; for 1.25h;
|
|
|
With Chloro-oxo-acetic acid; dimethyl sulfoxide; In dichloromethane; at -78 ℃; for 0.166667h;
6-methyl-3-pyridinemethanol; In dichloromethane; at -78 ℃; for 2.5h;
With triethylamine; In dichloromethane; at -78 - 20 ℃;
|
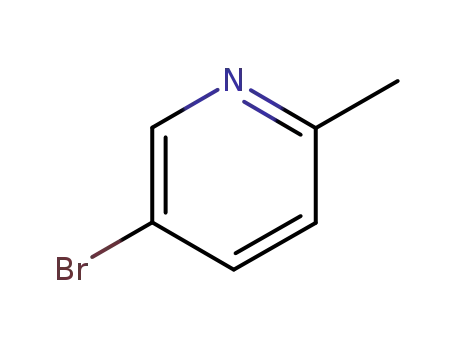
5-bromo-2-methylpyridine

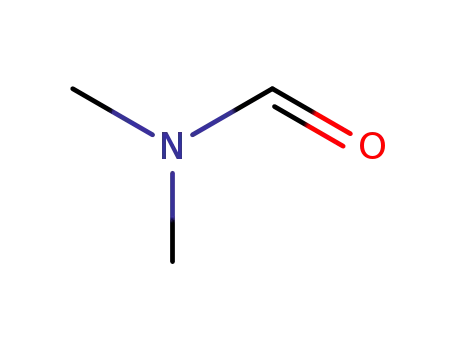
N,N-dimethyl-formamide


2-methyl-5-formylpyridine
| Conditions | Yield |
|---|---|
|
5-bromo-2-methylpyridine; With n-butyllithium; isopropylmagnesium chloride; In tetrahydrofuran; hexane; at -10 ℃; for 0.5h;
N,N-dimethyl-formamide; In tetrahydrofuran; at 0 ℃; for 2h;
|
85% |
|
5-bromo-2-methylpyridine; With n-butyllithium; In tetrahydrofuran; at -78 ℃; for 1h;
N,N-dimethyl-formamide; In tetrahydrofuran; at -78 ℃; for 1h;
|
72% |
|
5-bromo-2-methylpyridine; With n-butyllithium; In tetrahydrofuran; at -78 ℃; for 1h;
N,N-dimethyl-formamide; In tetrahydrofuran; at -78 ℃; for 1h;
|
72% |
|
5-bromo-2-methylpyridine; With n-butyllithium; isopropylmagnesium chloride; In tetrahydrofuran; hexane; at 10 ℃; for 1h;
N,N-dimethyl-formamide; In tetrahydrofuran; hexane; at 20 ℃; for 2h;
|
38% |
|
5-bromo-2-methylpyridine; With isopropylmagnesium chloride; In tetrahydrofuran; at 0 - 20 ℃; for 6h;
N,N-dimethyl-formamide; In tetrahydrofuran; at 0 - 20 ℃;
|
4.5 g |
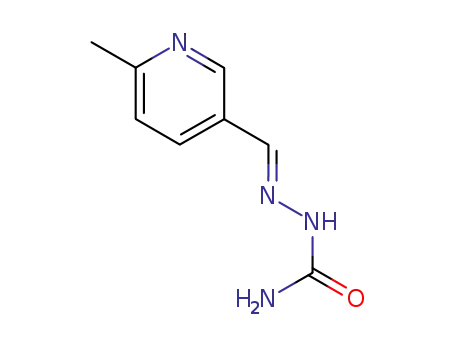
6-methyl-pyridine-3-carbaldehyde semicarbazone

6-methyl-3-pyridinemethanol
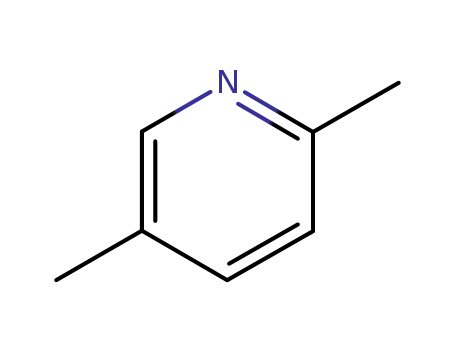
2,5-dimethylpyridine
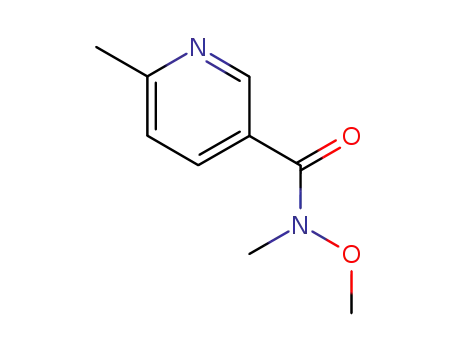
N-methoxy-N-methyl-6-methylnicotinamide
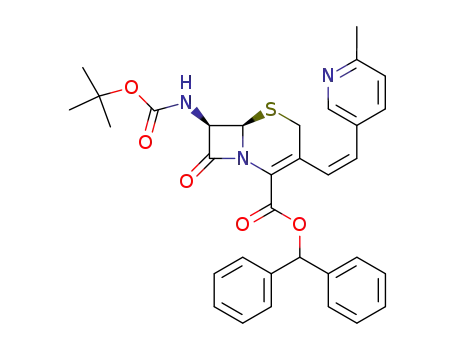
diphenylmethyl 7β-tert-butoxycarbonylamino-3-[(Z)-2-(6-methylpyridin-3-yl)vinyl]-3-cephem-4-carboxylate
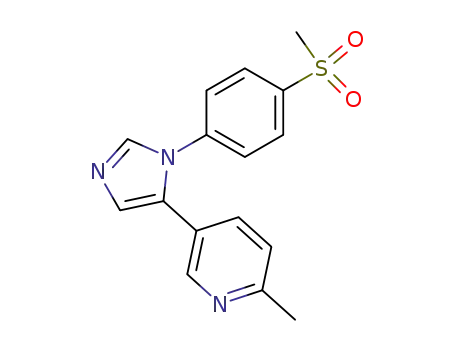
5-(6-Methyl-3-pyridyl)-1-(4-methylsulfonylphenyl)imidazole(73 % yield)
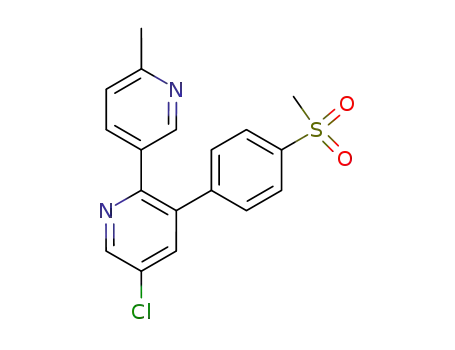
etoricoxib
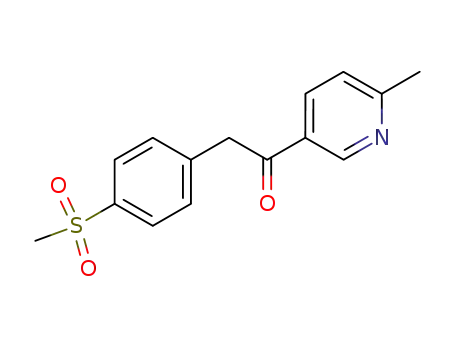
1-(6-methyl-3-pyridinyl)-2-[4-(methylsulfonyl)phenyl]ethanone