
CasNo: 85650-52-8
Molecular Formula: C17H19N3
Appearance: white solid
|
85650-52-8 Name |
|
|
Name |
Mirtazapine |
|
Synonym |
1,2,3,4,10,14B-HEXAHYDRO-2-METHYLPYRAZINO[2,1-A]PYRIDO[2,3-C][2]BENZAZEPINE;(rs)-1,2,3,4,10,14b-hexahydro-2-methylpyrazino-[2,1-a]pyrido[2,3-c][2]benzazepine;REMERON;ORG-3770;MIRTAZANINE;MIRTAZAPINE;MIRTAZEPINE;ZISPIN |
|
85650-52-8 Biological Activity |
|
|
Related Catalog |
Signaling Pathways >> GPCR/G Protein >> 5-HT Receptor Signaling Pathways >> Neuronal Signaling >> 5-HT Receptor Research Areas >> Neurological Disease |
|
85650-52-8 Chemical & Physical Properties |
|
|
Melting point |
114-116ºC |
|
Boiling point |
432.4±45.0 °C at 760 mmHg |
|
Density |
1.2±0.1 g/cm3 |
|
Molecular Formula |
C17H19N3 |
|
Molecular Weight |
265.353 |
|
Flash Point |
215.3±28.7 °C |
|
PSA |
19.37000 |
|
LogP |
2.75 |
|
Exact Mass |
265.157898 |
|
Vapour Pressure |
0.0±1.0 mmHg at 25°C |
|
Index of Refraction |
1.668 |
|
Storage condition |
Store at RT |
|
Water Solubility |
DMSO: ~8 mg/mL, soluble |
Mirtazapine, a noradrenergic and specific serotonergic antidepressant, is widely used in older people. The novel antidepressant mirtazapine has a dual mode of action. Mirtazapine is used primarily for the treatment of a major depressive disorder.
InChI:InChI=1/C17H19N3/c1-19-9-10-20-16(12-19)15-7-3-2-5-13(15)11-14-6-4-8-18-17(14)20/h2-8,16H,9-12H2,1H3/p+2/t16-/m0/s1
Mirtazapine (MRT) is an atypical antidepressant used to treat severe depression. MRT has a low oral bioavailability (about 50%) due to its low water solubility (BCS class II). The bioavailability study on rabbits’ plasma sample confirmed these results by showing a 1.34-fold increase in the drug oral absorption from the optimum MRT-PVP K-30 formula more than that of the plain MRT, with a relative bioavailability of 134.5%.
To provide a method capable of manufacturing a manufacturing intermediate of mirtazapine which is useful as an antidepressant at high yield and manufacturing high purity mirtazapine. SOLUTION: In a method for reacting 2-(4-methyl-2-phenyl-1-piperazinyl)-3-pyridinecarboxylic acid and hydrogenated bis(2-methoxyethoxy)aluminum sodium, manufacturing yield of 2-(4-methyl-2-phenyl-1-piperazinyl)-3-pyridine methanol is improved by washing a reaction mixture with alkali metal halide. Also in the method, by-production amount of dimer impurities during reaction can be suppressed and high quality mirtazapine can be manufactured in a method for reaction 2-(4-methyl-2-phenyl-1-piperazinyl)-3-pyridine methanol and sulfuric acid. SELECTED DRAWING: None COPYRIGHT: (C)2017,JPOandINPIT
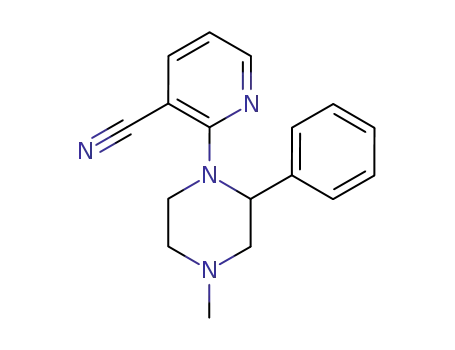
3-cyano-2-(4-methyl-2-phenyl-1-piperazynyl)pyridine

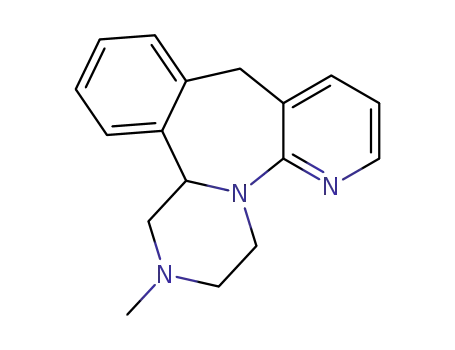
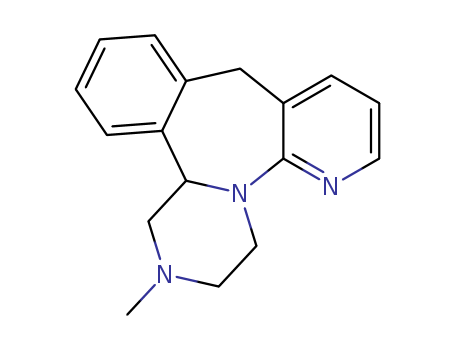
Mirtazapine
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 3 steps
1: 63 percent / sodium hydroxide / ethane-1,2-diol / 7 h / 130 °C
2: 97 percent / sodium bis(2-methoxyethoxy)aluminum hydride / toluene / 2 h / 55 - 60 °C
3: 91 percent / conc. sulfuric acid / CH2Cl2 / 3 h / Heating
With sodium hydroxide; sulfuric acid; sodium bis(2-methoxyethoxy)aluminium dihydride; In dichloromethane; ethylene glycol; toluene;
|
|
|
Multi-step reaction with 3 steps
1.1: potassium hydroxide; methanol / water / 90 °C
1.2: 0.5 h / 20 - 30 °C / pH ~ 7
2.1: sodium bis(2-methoxyethoxy)aluminium dihydride / toluene / 15 - 30 °C
2.2: 10 - 30 °C
2.3: 1 h / 30 - 60 °C
3.1: sulfuric acid / 15 - 30 °C
3.2: 30 °C / pH 10 - 11
With methanol; sulfuric acid; sodium bis(2-methoxyethoxy)aluminium dihydride; potassium hydroxide; In water; toluene;
|
|
|
Multi-step reaction with 3 steps
1: hydrogenchloride / ethanol; water; dichloromethane / 4 h / 100 °C / pH 7
2: sodium bis(2-methoxyethoxy)aluminium dihydride / tetrahydrofuran; toluene / 5 h / 10 - 40 °C / Inert atmosphere
3: sulfuric acid / 7 h / 15 - 55 °C / Inert atmosphere
With hydrogenchloride; sulfuric acid; sodium bis(2-methoxyethoxy)aluminium dihydride; In tetrahydrofuran; ethanol; dichloromethane; water; toluene;
|
|
|
Multi-step reaction with 3 steps
1: potassium hydroxide / ethanol / 20 h / 100 °C
2: lithium aluminium tetrahydride / tetrahydrofuran / 3 h / 5 °C / Reflux; Inert atmosphere
3: sulfuric acid / 9.41 h / 15 - 35 °C / Inert atmosphere
With lithium aluminium tetrahydride; sulfuric acid; potassium hydroxide; In tetrahydrofuran; ethanol;
|
|
|
Multi-step reaction with 3 steps
1: potassium hydroxide / ethanol / 4 h / 100 °C
2: sodium bis(2-methoxyethoxy)aluminium dihydride / tetrahydrofuran; toluene / 12.5 h / 25 °C
3: sulfuric acid / 7 h / 40 °C
With sulfuric acid; sodium bis(2-methoxyethoxy)aluminium dihydride; potassium hydroxide; In tetrahydrofuran; ethanol; toluene;
|
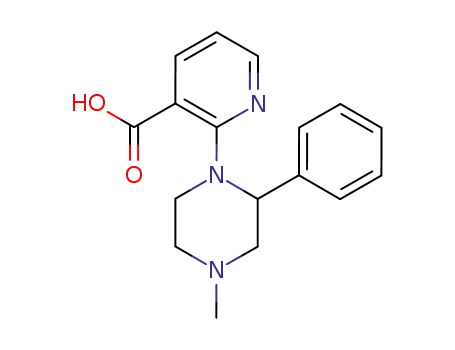
2-(4-methyl-2-phenyl-1-piperazinyl)-3-pyridinecarboxylic acid


Mirtazapine
| Conditions | Yield |
|---|---|
|
With sodium bis(2-methoxyethoxy)aluminium dihydride; In tetrahydrofuran; toluene; at 10 - 40 ℃; for 5h; Reagent/catalyst; Solvent; Inert atmosphere;
|
89% |
|
Multi-step reaction with 2 steps
1: 97 percent / sodium bis(2-methoxyethoxy)aluminum hydride / toluene / 2 h / 55 - 60 °C
2: 91 percent / conc. sulfuric acid / CH2Cl2 / 3 h / Heating
With sulfuric acid; sodium bis(2-methoxyethoxy)aluminium dihydride; In dichloromethane; toluene;
|
|
|
Multi-step reaction with 2 steps
1: sodium bis(2-methoxyethoxy)aluminium dihydride / tetrahydrofuran; toluene / 12.5 h / 25 °C
2: sulfuric acid / 7 h / 40 °C
With sulfuric acid; sodium bis(2-methoxyethoxy)aluminium dihydride; In tetrahydrofuran; toluene;
|
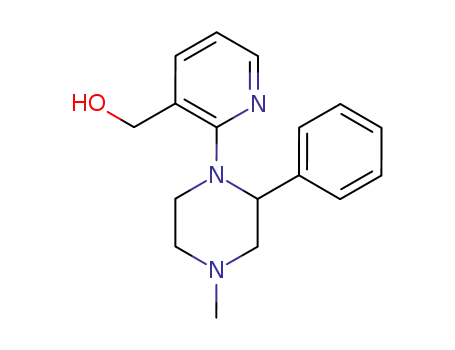
2-(4-methyl-2-phenylpiperazin-1-yl)-pyridine-3-methanol
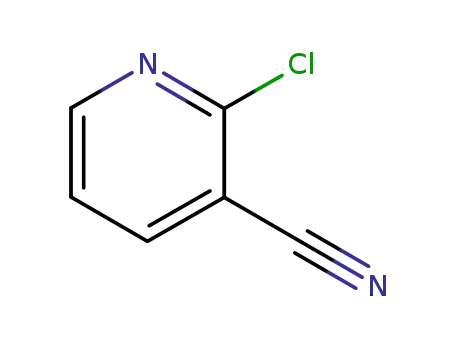
2-chloro-3-pyridinecarbonitrile
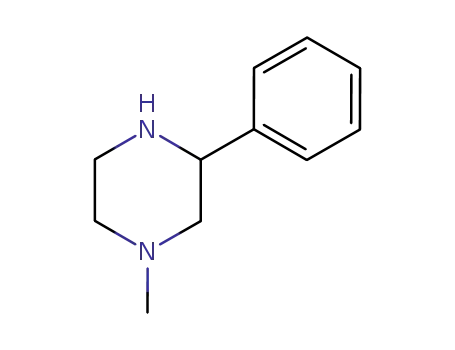
1-methyl-3-phenylpiperazine

2-(4-methyl-2-phenyl-1-piperazinyl)-3-pyridinecarboxylic acid
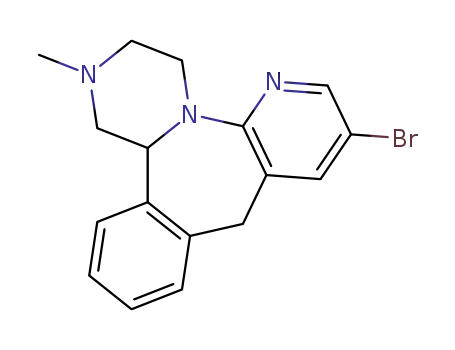
1,2,3,4,10,14b-hexahydro-8-bromo-2-methyl-pyrazino<2,1-a>pyrido<2,3-c><2>benzazepine
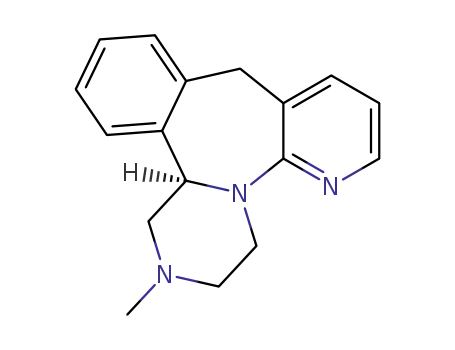
(-)-Mirtazapine
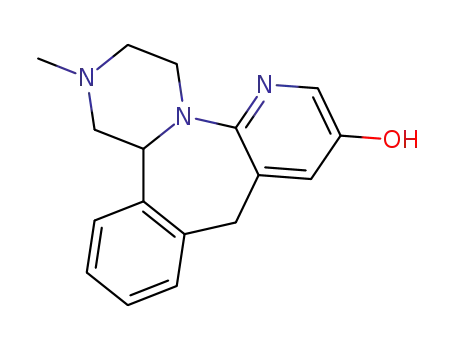
1,2,3,4,10,14b-hexahydro-2-methylpyrazino<2,1-a>pyrido<2,3-c><2>benzazepin-8-ol
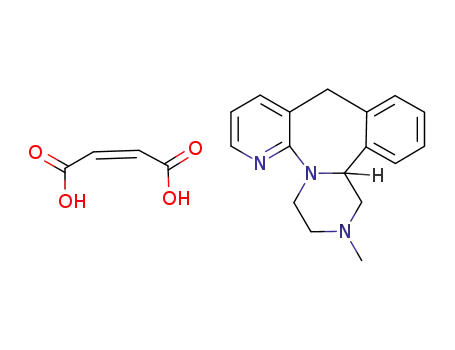
mirtazapinium hydrogenmaleate