Your Location:Home > Products > Pharmaceutical > Vonoprazan
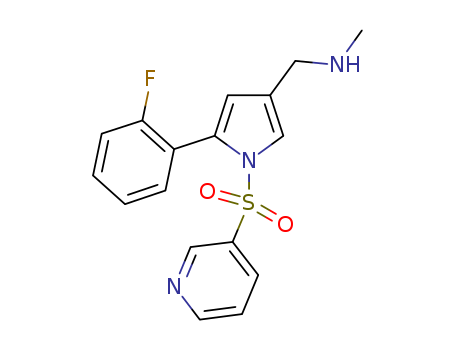

CasNo: 881681-00-1
Molecular Formula: C17H16FN3O2S
|
881681-00-1 Name |
|
|
Name |
Vonoprazan |
|
Synonym |
Vonoprazan;1-[5-(2-Fluorophenyl)-1-[(pyridin-3-yl)sulfonyl]-1H-pyrrol-3-yl]-N-methylmethanamine;1H-Pyrrole-3-methanamine,5-(2-fluorophenyl)-N-methyl-1-(3-pyridinylsulfonyl);TAK-438 (free base);Vonoprazan-025;Voronazan interfluoroisomer;Vonoprazan related Impuirty 29; Vonoprazan m-Fluoro Isomer |
|
881681-00-1 Biological Activity |
|
|
Related Catalog |
Signaling Pathways >> Membrane Transporter/Ion Channel >> Proton Pump Research Areas >> Endocrinology |
|
881681-00-1 Chemical & Physical Properties |
|
|
Boiling point |
530.3±60.0 °C at 760 mmHg |
|
Density |
1.3±0.1 g/cm3 |
|
Molecular Formula |
C17H16FN3O2S |
|
Molecular Weight |
345.391 |
|
Flash Point |
274.5±32.9 °C |
|
LogP |
2.74 |
|
Exact Mass |
345.094727 |
|
Vapour Pressure |
0.0±1.4 mmHg at 25°C |
|
Index of Refraction |
1.622 |
Vonoprazan, belongs to a new class of acid suppressant medications, the potassium-competitive acid blocker (P-CAB). Vanoprazan fumarate is a new oral gastric acidity drug developed by Takeda Pharmaceutical and Otsuka Pharmaceuticals. It is used to treat duodenal ulcers, gastric ulcers, reflux esophagitis, gastric ulcers or recurrent duodenal ulcers caused by low dosages of Aspirin, and Helicobacter pylori. It can also supplement treatment of gastric ulcers, duodenal ulcers, gastric MALT lymphoma, idiopathic thrombocytopenic purpura, early gastric cancer, and Helicobacter pylori infection gastritis. Vonoprazan (VPZ) inhibits gastric acid secretion more potently than proton pump inhibitors.
Vonoprazan, a novel acid-suppressive drug, is non-inferior to proton pump inhibitors (PPIs) for the management of gastric acid-related diseases. Vonoprazan has been shown to have effects on the hERG channel current at the doses studied with an IC50 value of 4.8 μg/ml. Compared to the traditional protein pump inhibitor Lansoprazole, Vonoprazan takes effect through competitive and reversible inhibition of K+ in protein pumps. Multiple clinical trials have proved that in cases of erosive esophagitis, Vonoprazan prevents and treats gastric and duodenal ulcers. Inform your doctor if you have the following conditions: kidney disease.
License data: US DailyMed: Vonoprazan
To elucidate the incidence and type of adverse events (AEs) in patients taking vonoprazan. Vonoprazan is well tolerated and shows similar safety compared to PPIs. The safety of vonoprazan may be primarily influenced by its indications and duration.
The invention discloses vonoprazan salt as well as a preparation method and application thereof, and the vonoprazan salt is salt formed by vonoprazan and a complex of organic acid and bismuth. Compared with the existing vonoprazan salt, the salt formed by the vonoprazan and the complex of organic acid and bismuth has many satisfactory advantages, such as good solubility and stability, improvement of bioavailability, synergistic increase of drug effect and the like.
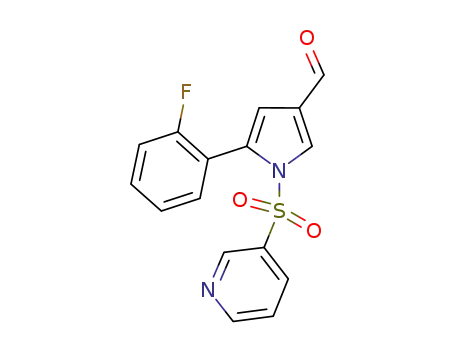
5‐(2-fluorophenyl)‐1‐(pyridine-3-sulfonyl)-1H-pyrrole-3-carbaldehyde

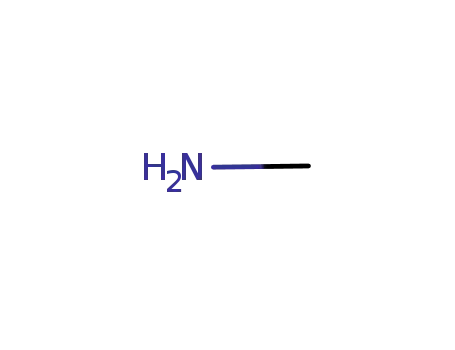
methylamine

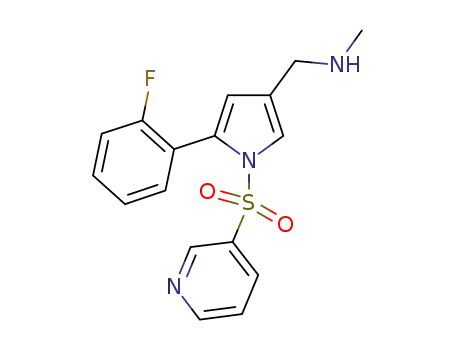
1-(5-(2-fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrole-3-yl)-N-methylmethanamine
| Conditions | Yield |
|---|---|
|
5‐(2-fluorophenyl)‐1‐(pyridine-3-sulfonyl)-1H-pyrrole-3-carbaldehyde; methylamine; In methanol; at 20 - 30 ℃; for 0.5h; Inert atmosphere;
With sodium tetrahydroborate; In methanol; N,N-dimethyl acetamide; at 0 - 5 ℃; for 1h;
|
80.2% |
|
5‐(2-fluorophenyl)‐1‐(pyridine-3-sulfonyl)-1H-pyrrole-3-carbaldehyde; methylamine; In methanol; isopropyl alcohol; at 10 ℃; for 2h;
With sodium tetrahydroborate; In methanol; isopropyl alcohol; at 0 - 5 ℃; for 1h; Temperature; Solvent;
|
64.07% |
|
With sodium cyanoborohydride; In methanol; at 20 ℃;
|
57% |
|
5‐(2-fluorophenyl)‐1‐(pyridine-3-sulfonyl)-1H-pyrrole-3-carbaldehyde; methylamine; In methanol; at 20 ℃; for 0.5h;
With sodium tetrahydroborate; In methanol; at 20 ℃; for 0.166667h;
With hydrogenchloride; water; sodium hydrogencarbonate; more than 3 stages;
|
|
|
5‐(2-fluorophenyl)‐1‐(pyridine-3-sulfonyl)-1H-pyrrole-3-carbaldehyde; methylamine; In methanol; at 20 ℃; for 0.5h;
With sodium tetrahydroborate; In methanol; at 20 ℃; for 0.166667h;
|
1.3 g |
|
5‐(2-fluorophenyl)‐1‐(pyridine-3-sulfonyl)-1H-pyrrole-3-carbaldehyde; methylamine; In methanol; at 10 - 35 ℃; for 0.5h;
With methanol; sodium tetrahydroborate; at 10 - 35 ℃; for 0.166667h;
|
1.3 g |
|
With 5%-palladium/activated carbon; hydrogen; ammonium bicarbonate; In methanol; at 17 - 30 ℃; for 4h; under 3000.3 Torr; Temperature; Pressure; Autoclave; Large scale;
|
1.8 kg |
|
5‐(2-fluorophenyl)‐1‐(pyridine-3-sulfonyl)-1H-pyrrole-3-carbaldehyde; methylamine; In methanol; ethanol; at 20 - 30 ℃; for 0.75h;
With pyridine; sodium tetrahydroborate; In methanol; ethanol; N,N-dimethyl acetamide; at -10 - 0 ℃; for 1h;
|
|
|
5‐(2-fluorophenyl)‐1‐(pyridine-3-sulfonyl)-1H-pyrrole-3-carbaldehyde; methylamine; In methanol; at 25 ℃; for 0.5h;
With methanol; sodium tetrahydroborate; In N,N-dimethyl acetamide; at -10 - -5 ℃; for 1h; Solvent; Temperature;
|
|
|
In methanol; at 20 ℃;
|
18.2 g |
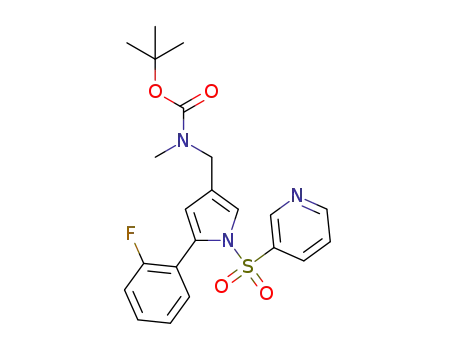
((5-(2-fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl)methyl)(methyl)carbamic acid tert-butyl ester


1-(5-(2-fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrole-3-yl)-N-methylmethanamine
| Conditions | Yield |
|---|---|
|
With sodium carbonate; trifluoroacetic acid; In dichloromethane; at 25 - 30 ℃; pH=8 - 9; Temperature;
|
86% |
|
With hydrogenchloride; In water; at 0 - 5 ℃; Reagent/catalyst;
|

5‐(2-fluorophenyl)‐1‐(pyridine-3-sulfonyl)-1H-pyrrole-3-carbaldehyde

methylamine
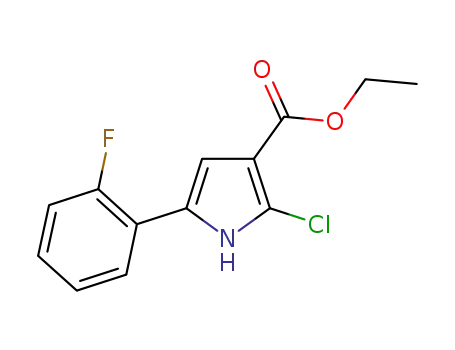
ethyl 2-chloro-5-(2-fluorophenyl)-1H-pyrrole-3-carboxylate
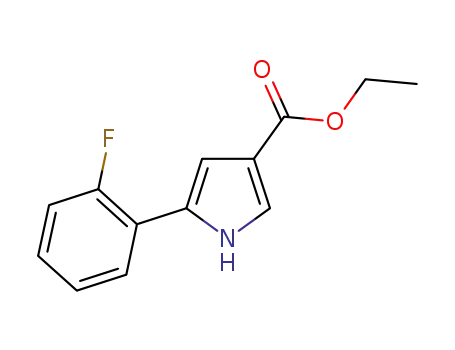
5-(2-fluorophenyl)-1H-pyrrole-3-carboxylic acid ethyl ester