- 86-21-56469616
- 86-18019205509
- minstar@minstargroup.com
- Language:English
- English
Your Location:Home >Products >Pharmaceutical >51264-14-3
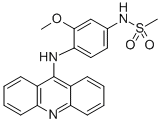

Appearance:powder
Throughput:100|Kilogram|Day
pd_productuse:intermediate
Delivery Time:in stock
Purity:98% purity
Amsacrine Chemical Properties
Melting point 230-240 °C
Boiling point 563.0±60.0 °C(Predicted)
density 1.2205 (rough estimate)
refractive index 1.6740 (estimate)
storage temp. Keep in dark place,Sealed in dry,2-8°C
pka 8.55±0.10(Predicted)
CAS DataBase Reference 51264-14-3
IARC 2B (Vol. 76) 2000
Safety Information
RIDADR 3249
HazardClass 6.1(b)
PackingGroup III
Hazardous Substances Data 51264-14-3(Hazardous Substances Data)
Toxicity LD50 in male, female CDF1 mice: 810 mg/m2; 729 mg/m2 orally (Pavkov)
MSDS Information
Amsacrine Usage And Synthesis
Description Amsacrine is a cytostatic reported to be active against adult lymphoblastic leukemia which has failed primary treatment or become resistant. Its clinical use, however, is associated with significant neuro-, gastro- and hepatotoxicity.
Originator Auckland Cancer Chemotherapy Lab (New Zealand)
Uses Amsacrine (Amsidyl) is used as an Investigational drug.
Uses Amsacrine is a drug undergoing intensive trials for severe leukemia and lymphoma. It is a cytotoxic drug that binds with DNA with expressed specificity to the adenosine–tyrosine pair, thus inhibiting DNA synthesis. It has been suggested to be used for severe leukemia. A synonym of this drug is amsidyl.
Uses Antineoplastic.
Definition ChEBI: A sulfonamide that is N-phenylmethanesulfonamide substituted by a methoxy group at position 3 and an acridin-9-ylamino group at position 4. It exhibits antineoplastic activity.
Brand name Amsidyl (Parke-Davis);Amsakrin.
Chemical Synthesis Amsacrine, 4-(9-acridinylamino)-3-methoxyphenyl-N-methansulfonamide (30.6.11), is made by sulfonating 4-nitro-m-anisidine with methanesulfonyl chloride, which forms a sulfonyl amide 30.6.9, and the nitro group is reduced to an amino group by hydrogen, forming 4-amino-3-methoxyphenyl-N-methansulfonamide (30.6.10). Reacting this with 9-chloroacridine gives amsacrine (30.6.11).
Amsacrine Preparation Products And Raw materials
Raw materials 4-Nitroaniline-->Methanesulfonyl chloride-->Methanesulfonamide-->9-Chloroacridine-->Amsacrine hydrochloride
CAS:1257044-40-8
CAS:881681-00-1
CAS:4920-80-3
CAS:116622-38-9