Your Location:Home > Products > Fine Chemicals > Fisetin
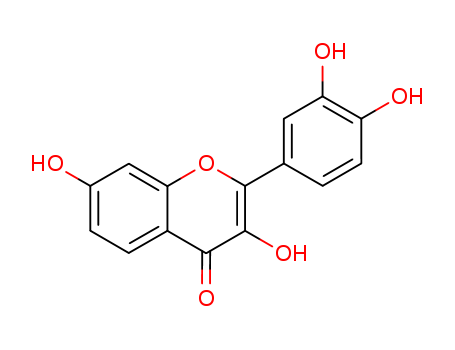

CasNo: 528-48-3
Molecular Formula: C15H10O6
Appearance: yellow to brown crystalline powder
|
528-48-3 Name |
|
|
Name |
Fisetin |
|
Synonym |
fisetholz;fustel;fustet;jungerfustik;superfustel;superfustelk;ungarischesgelbholz;3,7,3',4'-TETRAHYDROXYFLAVONE |
|
528-48-3 Biological Activity |
|
|
Related Catalog |
Signaling Pathways >> Cell Cycle/DNA Damage >> PPAR Signaling Pathways >> Cell Cycle/DNA Damage >> Sirtuin Signaling Pathways >> Epigenetics >> Sirtuin Signaling Pathways >> Apoptosis >> TNF Receptor Natural Products >> Flavonoids Research Areas >> Cancer Research Areas >> Inflammation/Immunology Research Areas >> Metabolic Disease Research Areas >> Neurological Disease |
|
Target |
Sirtuin, PPAR, TNF-alpha[1][2] |
|
528-48-3 Chemical & Physical Properties |
|
|
Melting point |
330ºC |
|
Boiling point |
599.4±50.0 °C at 760 mmHg |
|
Density |
1.7±0.1 g/cm3 |
|
Molecular Formula |
C15H10O6 |
|
Molecular Weight |
286.236 |
|
Flash Point |
233.0±23.6 °C |
|
PSA |
111.13000 |
|
LogP |
2.52 |
|
Exact Mass |
286.047729 |
|
Vapour Pressure |
0.0±1.8 mmHg at 25°C |
|
Index of Refraction |
1.785 |
|
Storage condition |
−20°C |
Fisetin is a plant polyphenol from the flavonoid group. It is used in biological studies as spleen tyrosine kinase inhibitors for autoimmune inflammation disease. Fisetin belongs to a class of flavonoids, which is found abundantly in several fruits and vegetables. Fisetin is isolated from many fruits and vegetables and has been confirmed to improve airway hyperresponsiveness in asthmatic mice. This compound has neuroprotective properties.The exact value can be found on the certificate. Produced by PhytoLab GmbH & Co. KG
InChI:InChI=1/C15H10O6/c16-8-2-3-9-12(6-8)21-15(14(20)13(9)19)7-1-4-10(17)11(18)5-7/h1-6,16-18,20H
Fisetin is one of the most popular flavonoids found in vegetables and fruits with several health benefits such as antidiabetic, anticancer, and anti-inflammatory activities. Biotechnology or chemosynthesis tools were used for the synthesis of fisetin and its analogs to improve their yield and efficacy. One pharmacokinetic study has shown that fisetin was absorbed quickly after intravenous (i.v.) administration (10 mg/kg) via tails vein in Sprague-Dawley rats and emerged into glucuronides and sulfates.
Cancer is one of the major causes of mortality, globally. This review aims to provide in-depth information regarding fisetin as a potential candidate for anticancer therapy, its properties and various formulation strategies.
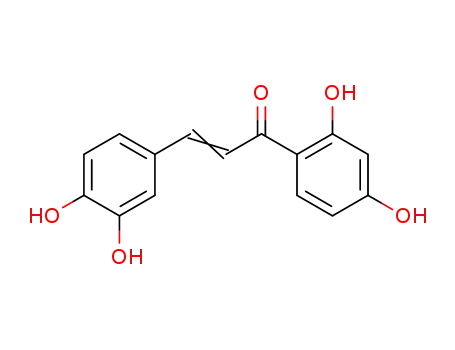
3,4,2',4'-tetrahydroxy-chalcone

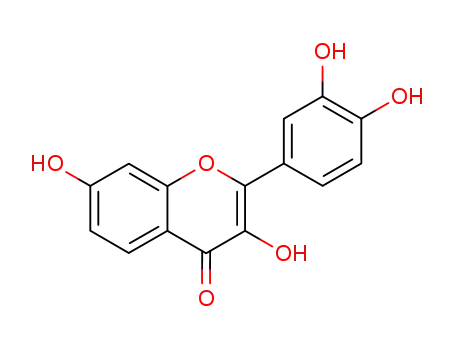
3,7,3',4'-tetrahydroxyflavone
| Conditions | Yield |
|---|---|
|
With sodium carbonate / sodium bicarbonate buffer solution; potassium hydrogen sulfate complex; In dichloromethane; acetone; at 20 ℃; for 21h;
|
96.7% |
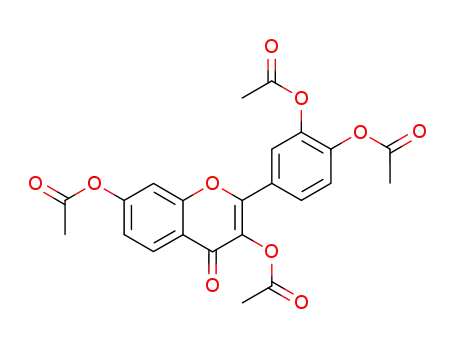
3,3′,4′,7-tetraacetoxyflavone


3,7,3',4'-tetrahydroxyflavone
| Conditions | Yield |
|---|---|
|
With sodium dithionite; water; sodium hydroxide; In methanol; at 20 ℃; for 4h; Inert atmosphere;
|
65% |
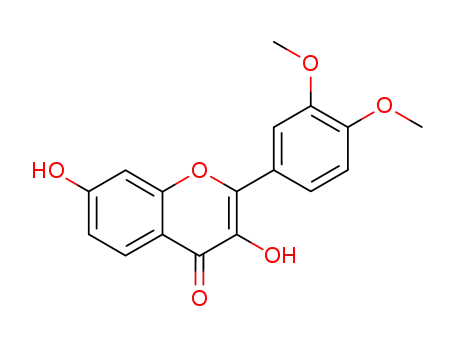
Fisetin 3',4'-dimethyl ether
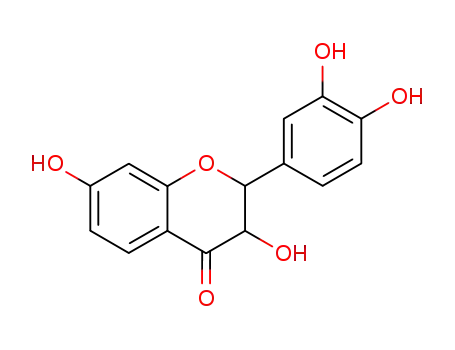
3,7,3',4'-tetrahydroxyflavanone
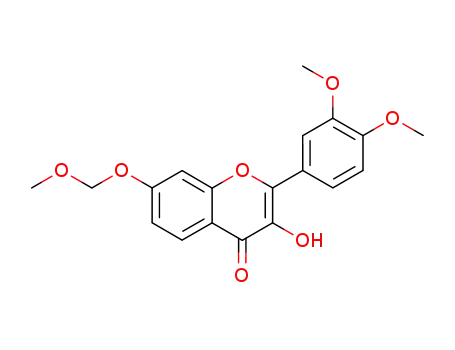
2-(3,4-dimethoxy-phenyl)-3-hydroxy-7-methoxymethoxy-chromen-4-one
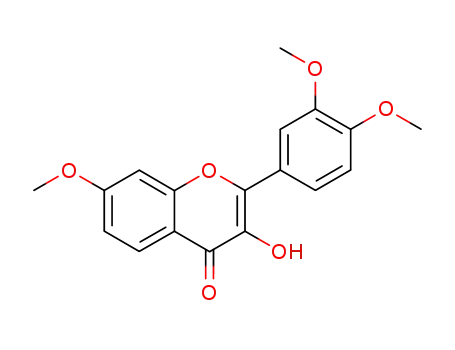
2-(3,4-dimethoxyphenyl)-3-hydroxy-7-methoxy-4H-chromen-4-one
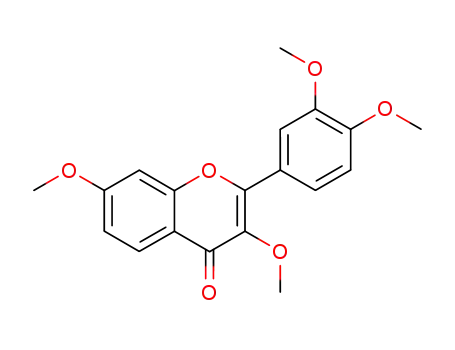
3,3',4',7-tetra-O-methylfisetin

3,3′,4′,7-tetraacetoxyflavone
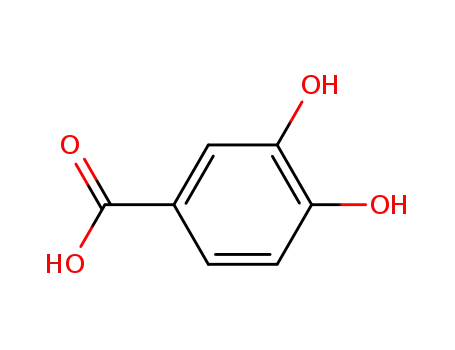
3,4-Dihydroxybenzoic acid
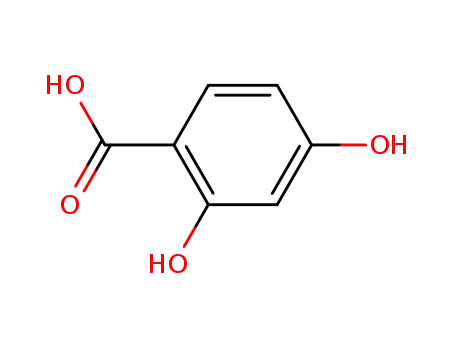
4-hydroxysalicylic acid