Your Location:Home > Products > Fine Chemicals > 5-Aminolevulinic acid hydrochloride
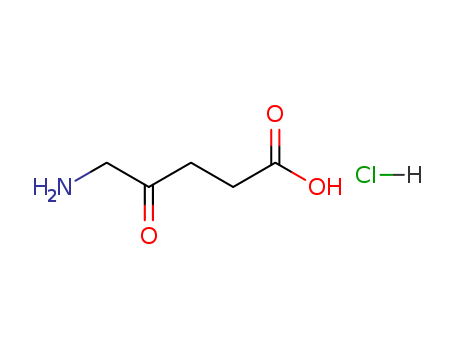

CasNo: 5451-09-2
Molecular Formula: C5H10ClNO3
Appearance: white to pale yellow crystals
|
5451-09-2 Name |
|
|
Name |
5-Aminolevulinic acid hydrochloride |
|
Synonym |
5-AminoL;evuL;inic acid hydrochL;TIMTEC-BB SBB003880;DELTA-AMINOLEVULINIC ACID HYDROCHLORIDE CELL CULTUR;5-AminolevulinicAcidHydrochloride(5-Ala);5-Ala;5-Aminolevulinicacidhydrochloride |
|
5451-09-2 Biological Activity |
|
|
Related Catalog |
Signaling Pathways >> Autophagy >> Autophagy Signaling Pathways >> Autophagy >> Mitophagy Research Areas >> Metabolic Disease Natural Products >> Others |
|
References |
[1]. Stummer, W., et al., Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol, 2006. 7(5): p. 392-401. [2]. Eyupoglu, I.Y., et al., Improving the extent of malignant glioma resection by dual intraoperative visualization approach. PLoS One, 2012. 7(9): p. e44885. |
|
5451-09-2 Chemical & Physical Properties |
|
|
Melting point |
~150 °C (dec.) |
|
Boiling point |
311.5±27.0 °C at 760 mmHg |
|
Density |
1.2±0.1 g/cm3 |
|
Molecular Formula |
C5H10ClNO3 |
|
Molecular Weight |
167.59 |
|
Flash Point |
142.2±23.7 °C |
|
PSA |
80.39000 |
|
LogP |
-0.79 |
|
Appearance of Characters |
powder |
|
Vapour Pressure |
0.0±1.4 mmHg at 25°C |
|
Index of Refraction |
1.482 |
|
Storage condition |
2-8°C |
|
Water Solubility |
H2O: 50 mg/mL |
5-aminolevulinic acid (5-ALA), an endogenous non-proteinogenic five-carbon amino acid, is the indispensable intermediate which involves in the tetrapyrrole biosynthesis and is the first compound from the porphyrin synthesis to heme. 5-Aminolevulinic acid hydrochloride is white to pale yellow crystals. Aminolevulinic acid HCl (5-aminolevulinic acid HCl; ALA) is a prodrug that is metabolized intracellularly to form the photosensitizing molecule protoporphyrin (PpIX). 5-Aminolevulinic acid hydrochloride has been used as a supplement for culturing Escherichia coli cells for heme biosynthesis.
InChI:InChI=1/C5H9NO3.ClH/c6-3-4(7)1-2-5(8)9;/h1-3,6H2,(H,8,9);1H
Oral administration of 5-aminolevulinic acid hydrochloride (5-ALA-HCl) has been reported to enhance the hypotensive effects associated with anesthetics, especially in elderly hypertensive patients treated with antihypertensive agents. Administration of 5-ALA is considered to enhance the effects of antihypertensive agents- and anesthetics-induced hypotension; reportedly via the binding of protoporphyrin IX from 5-ALA to soluble guanylate cyclase, with resultant increase in cGMP and concomitant vasodilation.
Patient selection for transurethral resection of the bladder tumor using photodynamic diagnosis (PDD-TURBT) with oral 5-aminolevulinic acid (5-ALA) hydrochloride for non-muscle-invasive bladder cancer (NMIBC) is still unclear as to the best balance of risks (adverse events including hypotension) and benefits (reduction of intravesical recurrence). In this study, PDD-TURBT with oral 5-ALA hydrochloride for NMIBC resulted in significantly better short-term intravesical recurrence-free survival than WL-TURBT. Intravesical recurrence-free survival within 500 days was significantly better in the PDD-TURBT cases than in the WL-TURBT cases.

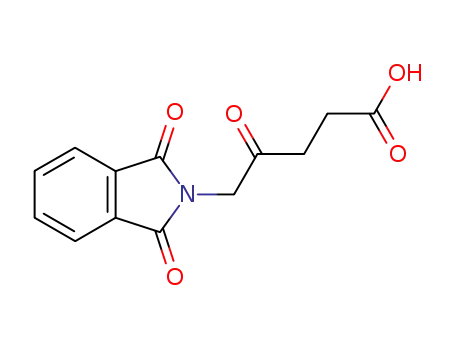
5-phthalimidyl levulinic acid

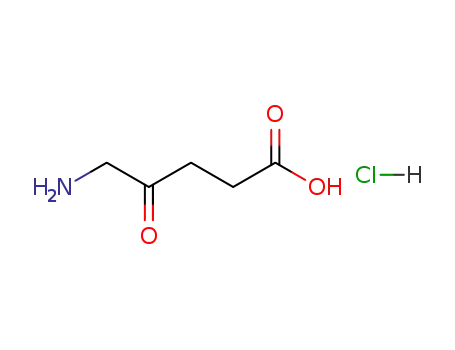
5-aminolevulinic acid hydrochloride
| Conditions | Yield |
|---|---|
|
|

5-phthalimidyl levulinic acid


5-aminolevulinic acid hydrochloride
| Conditions | Yield |
|---|---|
|
With hydrogenchloride; In water; for 6h; Reflux;
|
96.6% |
|
With hydrogenchloride; In water; for 10h; Reflux;
|
85% |
|
With hydrogenchloride; In water; for 16h; Reflux;
|
83% |
|
With hydrogenchloride; for 8h; Heating;
|
63.8% |
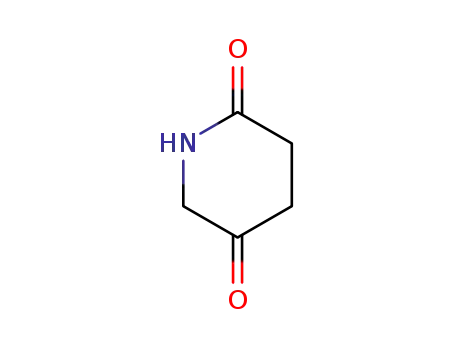
piperidine-2,5-dione

5-phthalimidyl levulinic acid
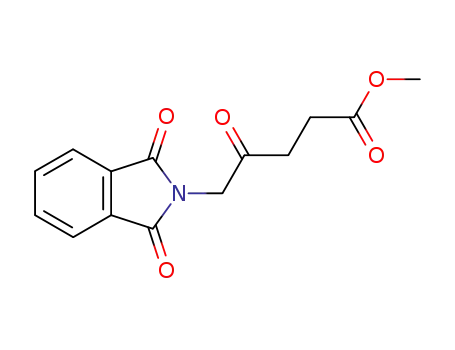
methyl 5-(1,3-dioxoisoindolin-2-yl)-4-oxopentanoate
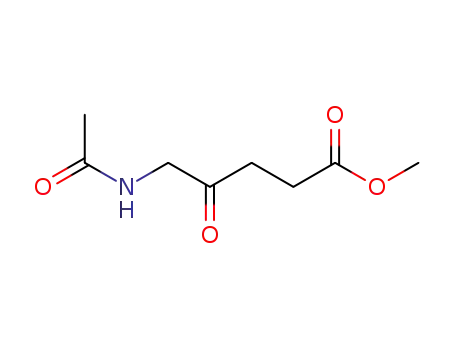
N-acetyl-5-aminolevulinic acid methyl ester
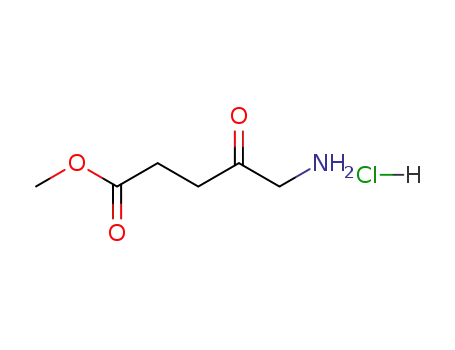
methyl aminolevulinate
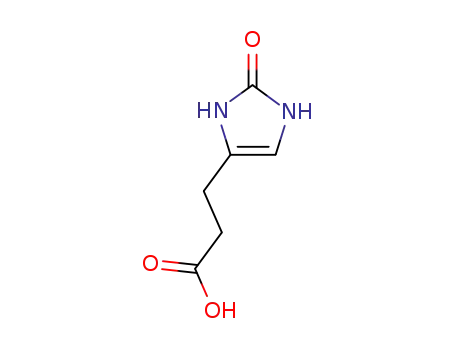
4-(2-carboxyethyl)imidazolin-2-one
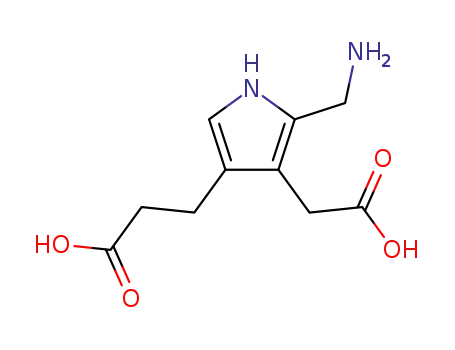
porphobilinogen
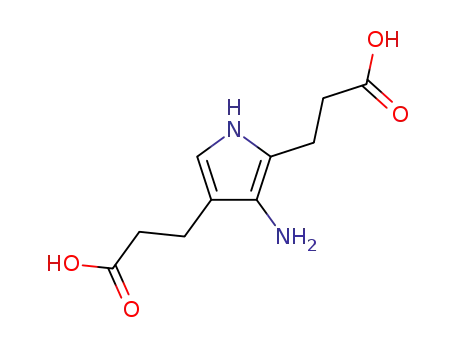
pseudo-porphobilinogen