Your Location:Home > Products > Fine Chemicals > 2-Ethyl-2-adamantanol


CasNo: 14648-57-8
Molecular Formula: C12H20O
|
14648-57-8 Name |
|
|
Name |
2-Ethyl-2-adamantanol |
|
Synonym |
2-ETHYL-2-HYDROXYTRICYCLO[3,3,1,1(3,7)])DECANE;2-ETHYL-2-HYDROXYADAMANTANE;2-Ethyl-2-hydroxyadamantane 95+%;2-Ethyl-2-adamantanol 14648-57-8;2-Ethyl-2-adamantanol 99.9% In stock;2-Ethyl-2-adamantanol ,99%;2-Ethyl-2-adamantane;2-Ethyl-2-adamantanol |
|
14648-57-8 Chemical & Physical Properties |
|
|
Melting point |
70 °C |
|
Boiling point |
265.5±8.0 °C at 760 mmHg |
|
Density |
1.0±0.1 g/cm3 |
|
Molecular Formula |
C12H20O |
|
Molecular Weight |
180.287 |
|
Flash Point |
110.2±10.9 °C |
|
PSA |
20.23000 |
|
LogP |
3.25 |
|
Exact Mass |
180.151413 |
|
Vapour Pressure |
0.0±1.2 mmHg at 25°C |
|
Index of Refraction |
1.527 |
2-Ethyl-2-adamantanol is White crystal. The reaction of 2-ethyl-2-adamantanol with the same reagent yields a mixture of compounds from which 2-adamantanone, 2-(1-acetoxyethyl)-2- adamantanol, and products of stabilization of 2-(1-nitroethyl)-2-adamantyl cation were isolated.
InChI:InChI=1/C12H20O/c1-2-12(13)10-4-8-3-9(6-10)7-11(12)5-8/h8-11,13H,2-7H2,1H3
Commercially available 2-ethyl-2-adamantanol was dehydrated and hydrogenated to yield EA. The alkyl diamondoids described in this report are the first examples of multicyclic hydrocarbons that combine extraordinary densities (>0.9 g/mL) with DCNs comparable to or exceeding that of conventional diesel fuel.
1. The relative binding affinities suggested that the rigid carbon framework provided by the pyrrolidine or piperidine rings results in a more favorable orientation inside the M2 channel pore as compared to large, freely rotating alkyl groups. The aminoadamantane derivatives exhibited similar NMDA antagonistic activity to amantadine 1. A striking finding was the antiviral activity of the adamantanols 4, and 6, which lack any NMDA antagonist activity.
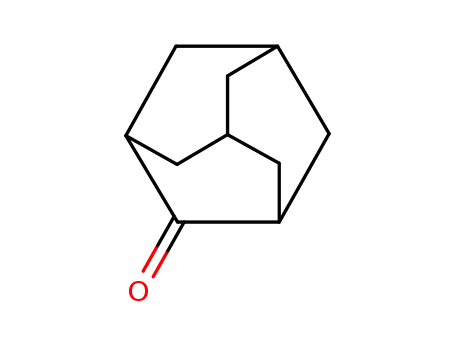
2-Adamantanone

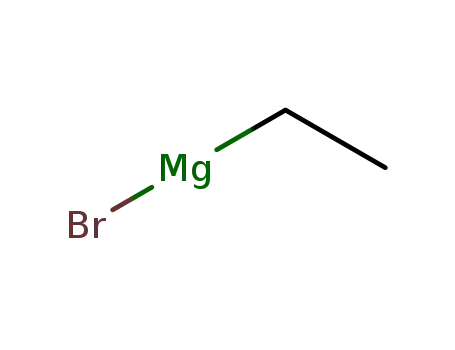
ethylmagnesium bromide

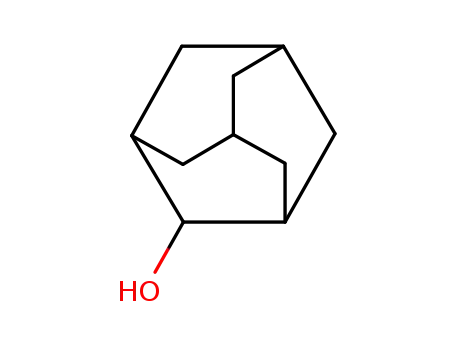
1-adamantanol

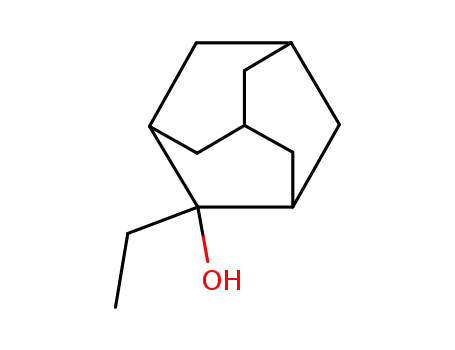
2-ethyl-2-adamantanol
| Conditions | Yield |
|---|---|
|
ethylmagnesium bromide; With (trimethylsilyl)methylmagnesium chloride; lithium chloride; zinc(II) chloride; In tetrahydrofuran; diethyl ether; at 20 ℃;
2-Adamantanone; In tetrahydrofuran; diethyl ether; at 0 ℃; for 3h;
|
80% 16% |
|
In tetrahydrofuran; diethyl ether; at 0 ℃;
|
80% 18% |
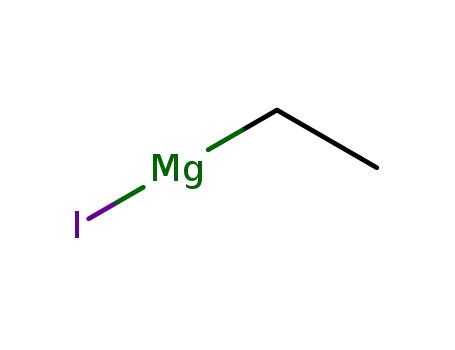
ethylmagnesium iodide


2-Adamantanone

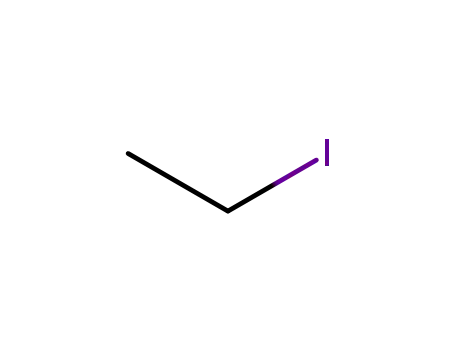
ethyl iodide


1-adamantanol


2-ethyl-2-adamantanol
| Conditions | Yield |
|---|---|
|
With magnesium; In tetrahydrofuran;
|

2-Adamantanone
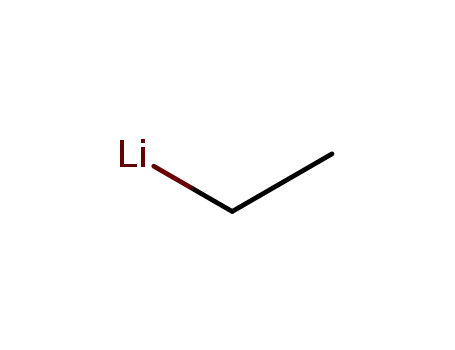
ethyllithium

ethylmagnesium iodide

ethyl iodide
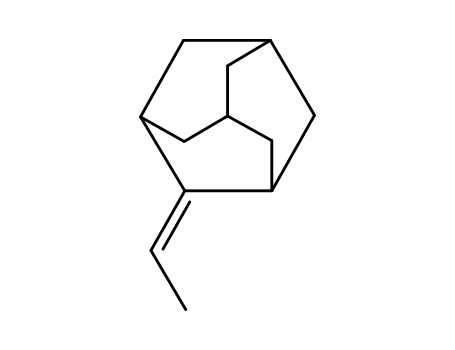
(1r,3r,5R*,7S*)-2-ethylideneadamantane

2-Adamantanone
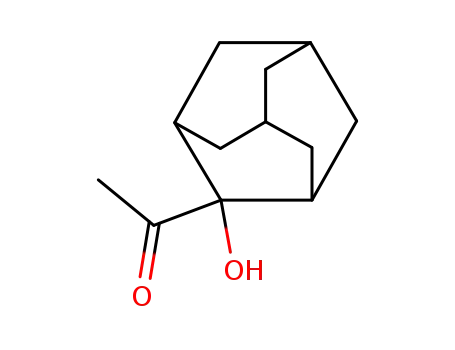
2-Hydroxy-2-adamantyl methyl ketone
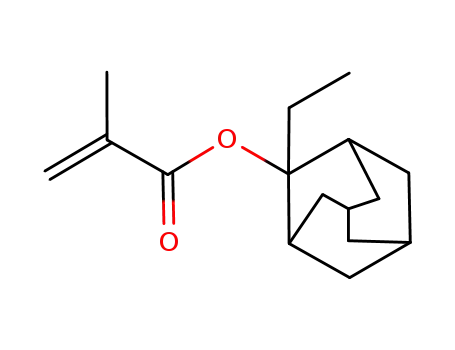
2-ethyladamantan-2-yl methacrylate