
CasNo: 171596-29-5
Molecular Formula: C22H19N3O4
Appearance: white to off-white cyrstalline solid
Indications and uses
Cialis (or Tadalafil) was developed by American pharmaceutical company Lilly. It is used to treat erectile dysfunction and belongs to a second generation of PDE5 inhibitors. Studies show that Cialis works very quickly, taking effect in around 15-20 minutes, and has a prolonged effect that can last for up to 36 hours. T1/2 is 17.5h.
Clinical Research
In a study of 348 cases of mild to severe erectile dysfunction, patients were randomly given 20mg of Cialis or a placebo. Results showed that in comparison to the placebo group, patients who took Cialis experienced improved intercourse success in the 24-36 hours following medication, with many patients achieving successful sexual intercourse twice in 36 hours. Side effect rate and severity were also no different from those of the placebo group. Over 5% of patients in the Cialis group experienced headaches and indigestion.
Side effects
No severe negative reactions. No perceived facial flushing or sight abnormalities following medication. Rare cases of headache and indigestion.
Warnings and precautions
Patients currently taking nitrates, experiencing angina pectoris, suffering from heart disease, patients who have unregulated hypertension or hypotension, or patients who have had a stroke in the past 6 months should not take Cialis.
Description
Tadalafil (market name “Cialis” or “Adcirca”) is a kind of PDE5 inhibitor used for the treatment of erectile dysfunction, benign prostatic hypertrophy and pulmonary arterial hypertension. The effect of Tadalafil is relaxing the blood vessels muscles and increasing the blood flow into the corpus cavernosum. The mechanism of action of tadalafil is through inhibiting the activity of the cGMP specific phosphodiesterase type 5 (PDE5). PDE5 degrades cGMP in the corpus cavernosum located around the penis. Therefore, tadalafi leads to the increased concentration of cGMP which further causes the smooth muscle relaxation and increased blood flow into the corpus cavernosum. Some clinical studies also implied that tadalafil could improve endothelia function in men with increased cardiovascular risk and lower the urinary tract symptoms secondary to benign prostatic hyperplasia.
Chemical Properties
White to Off-White Cyrstalline Solid
Originator
Lilly/ICOS (US)
Uses
analgesic, norepinephrine uptake blocker, mu-opiod receptor agonist.A phosphodiesterase 5 inhibitor.Tadalafil is used for the treatment of erectile dysfunction.
Uses
Tadalafil is used for the treatment of erectile dysfunction. A phosphodiesterase 5 inhibitor.
Definition
ChEBI: A pyrazinopyridoindole that is 2,3,6,7,12,12a-hexahydropyrazino[1',2':1,6]pyrido[3,4-b]indole-1,4-dione substituted at position 2 by a methyl group and at position 6 by a 1,3-benzodioxol-5-yl group (the 6R,12aR
Brand name
Cialis (Lilly).
General Description
Tadalafil, (6R-trans)-6-(1,3-benzodioxol-5-yl)-2,3,6,7,12,12a-hexahydro-2-methyl-pyrazino[1', 2' :1,6]pyrido[3,4-b]indole-1,4-dione (Cialis), is a potent PDE5 inhibitor.It received FDA approval for the treatment of erectiledysfunction in December 2003. Because of its half-life of17.5 hours, it is marketed as a 36-hour treatment. Tadalafil ispredominantly metabolized by hepatic enzymes, includingCYP3A4. The concomitant use of CYP3A4 inhibitors suchas ritonavir, indinavir, ketoconazole, as well as moderateCYP3A inhibitors such as erythromycin have been shown toresult in significant increases in tadalafil plasma levels.Much like sildenafil, tadalafil is under clinical investigationfor managing PAH.
Mechanism of action
Tadalafil was the last agent to be released and can be taken on a full stomach without slowing the onset. It has a much longer duration of action, lasting up to 48 hours, compared with sildenafil and vardenafil, which last for approximately 4 hours. The longer half-life of tadalafil results in a lengthened period of responsiveness as compared to sildenafil and vardenafil. This longer therapeutic window requires fewer time constraints for the effectiveness of tadalafil and has been interpreted as being advantageous through providing the option for more spontaneous sexual activity. Because of its long half-life, however, tadalafil, has been detected in plasma even 5 days after oral administration. This suggests the possibility of accumulation if taken regularly and in short intervals, which may result in an increased risk of side effects with the excessive use of this PDE5 inhibitor. The 3,4-methylenedioxy substitution on the phenyl ring was significant for increasing its potency as PDE5 inhibitor. Optimization of the chain on the piperazinedione ring resulted in no significant change in IC50s. Tadalafil is a highly potent PDE5 inhibitor (IC50, 5 nM), with high selectivity for PDE5 versus PDE1 through PDE4. The PDE5/PDE6 selectivity ratio is 85.
Pharmacokinetics
Tadalafil is different in structure from both sildenafil and vardenafil. It is rapidly absorbed and peaks in concentration (378 μg/L after a 20-mg dose) after 2 hours, displaying a long half-life of 17.5 hours. It also is metabolized by the liver (CYP3A4). Notably, its pharmacokinetics is not clinically influenced by alcohol or food intake or by factors such as diabetes or impaired hepatic or renal function.
Clinical Use
Tadalafil is one of the two new PDE5 inhibitors launched for the oral treatment of male erectile dysfunction. Tadalafil is a b-carboline derivative and it is structurally distinct from vardenafil (Levitraw) and sildenafil (Viagraw), both of which are PDE5 inhibitors based on a fused pyrimidine core structure. Tadalafil is synthesized in three steps starting from D-tryptophan methyl ester, by first condensing with piperonal in a Pictet-Spengler cyclization reaction to form the tetrahydro-β-carboline derivative, which is followed by chloroacetylation of the piperidine ring nitrogen and cyclization with methylamine. Tadalafil is a potent and highly selective inhibitor of PDE5 (IC50=1 nm). It shows >10,000-fold selectivity for PDE5 versus PDE1, 2, 3, 4, 7, 8 and 9, and >700-fold selectivity versus PDE6. Typically administered at 10 and 20 mg doses, tadalafil is rapidly absorbed and has a tmax of 2 h, which is slightly longer than those of sildenafil (1 h) and vardenafil (0.75 h). Clinically, all of these agents appear to have efficacy for many men within 30–60 min. However, tadalafil distinguishes itself from other PDE5 inhibitors in terms of significantly longer duration of action. The half-life of tadalafil dosed at 20 mg is 17.5 h as compared with 3.8 h for sildenafil (100 mg) and 4.7 h for vardenafil (20 mg). In clinical studies, significant rates of response were reported up to 36 h following drug ingestion. Tadalafil is predominantly metabolized in the liver by CYP3A4 to entities that are not active against PDE5 and excreted mainly as metabolites in the feces and the urine. The pharmacokinetics of tadalafil are unaffected by factors such as intake of food and alcohol, age, the presence of diabetes, and mild or moderate hepatic insufficiency. The most common drug-related adverse events are headache, back pain, dyspepsia, and myalgia. At 10 and 20 mg doses, Tadalafil does not have a significant effect on blood pressure and heart rate and does not result in increased instances of myocardial infarction. Rare reports of prolonged erections greater than 4 h and priapism have been noted with the use of tadalafil. Priapism, if not treated properly, can result in irreversible damage to the erectile tissue. Patients who have an erection lasting greater than 4 h are advised to seek emergency medical attention. Tadalafil has a modest synergistic effect on the nitrate-induced reduction in blood pressure and, as with sildenafil and vardenafil, it is contraindicated for use in patients on nitrate therapy. In diabetic patients, improvement of erectile function by tadalafil is irrespective of the type of diabetes and the type of diabetic therapy.
Side effects
Adverse effects related to oral therapy for erectile function are primarily concerned with adverse cardiovascular effects, because PDE5 inhibitors promote vasodilation and, therefore, have an inherent potential to cause hypotension. This is a particular concern for elderly patients with a preexisting condition.
Synthesis
D-(-)-Tryptophan methyl ester (175) and 1,3- benzodioxole-5-carboxaldehyde (176) were subjected to a modified Pictet-Spengler reaction to form cis- and transtetrahydro- β-carboline tricyclic compounds. The ciscompound 177 was isolated as a white solid in 42% yield. The basic nitrogen in the piperidine ring of 177 was acylated with chloroacetyl chloride (179) to give compound 180 in 93% yield. Finally, the diketonepiperazine ring was formed by adding 180 to 33% methylamine in ethanol under refluxing conditions and yielded tadalafil (XXII) in 77% as a white solid.
Drug interactions
Potentially hazardous interactions with other drugs Alpha-blockers: enhanced hypotensive effect - avoid concomitant use. Antibacterials: concentration possibly increased by clarithromycin and erythromycin; concentration reduced by rifampicin - avoid. Antifungals: concentration increased by ketoconazole - avoid; concentration possibly increased by itraconazole. Antivirals: concentration possibly increased by fosamprenavir and indinavir; increased by ritonavir - avoid; increased risk of ventricular arrhythmias with saquinavir - avoid; avoid high doses of tadalafil with telaprevir. Cobicistat: concentration of tadalafil possibly increased - reduce dose of tadalafil. Nicorandil: possibly enhanced hypotensive effect - avoid concomitant use. Nitrates: enhanced hypotensive effect - avoid concomitant use. Riociguat: enhanced hypotensive effect - avoid concomitant use.
Metabolism
Tadalafil is metabolised in the liver mainly by the cytochrome P450 isoenzyme CYP3A4. The major metabolite, the methylcatechol glucuronide, is inactive. Tadalafil is excreted, mainly as metabolites, in the faeces (61% of the dose), and to a lesser extent the urine (36% of the dose).
References
https://www.drugs.com/tadalafil.html https://www.drugbank.ca/drugs/DB00820 Roehrborn, C. G., et al. "Tadalafil administered once daily for lower urinary tract symptoms secondary to benign prostatic hyperplasia: a dose finding study." Journal of Urology 180.4(2008):1228. Rosano, Giuseppe M. C., et al. "Chronic Treatment with Tadalafil Improves Endothelial Function in Men with Increased Cardiovascular Risk." European Urology 47.2(2005):214-222.
InChI:InChI=1/C22H23N3O3/c1-24-8-9-25-17(22(24)26)11-15-14-4-2-3-5-16(14)23-20(15)21(25)13-6-7-18-19(10-13)28-12-27-18/h2-7,10,15,17,20-21,23H,8-9,11-12H2,1H3/t15?,17-,20?,21-/m1/s1
The invention provides a preparation method of tadalafil, and relates to the technical field of medicines. The preparation method comprises the steps of taking D-cis-carboline hydrochloride as an initial raw material, carrying out acylating chlorination reaction on the D-cis-carboline hydrochloride and chloroacetyl chloride under the action of an acid-binding agent, carrying out primary refining on an obtained chloroacetylate intermediate crude product, carrying out aminolysis cyclization reaction on the obtained high-purity chloroacetylate intermediate and methylamine, adding a pulping solvent into the obtained tadalafil crude product for pulping, carrying out solid-liquid separation, washing the obtained solid product, and drying to obtain the high-purity tadalafil. Moreover, the D-cis-carline hydrochloride raw material is cheap, easy to obtain and stable in quality, the D-cis-carline hydrochloride raw material is used as a starting raw material, the production period of tadalafil is short, and the production cost is low.
The invention discloses a preparation method of tadalafil. According to the method, a compound I is adopted as a starting material, the tadalafil is obtained through condensation, Fisher indole synthesis reaction, Pictet-Spengler reaction, acylation and ring closing in sequence, and dangerous reagents (n-butyllithium and the like) are not used during ring closing. The reaction route is simple, the reaction condition is mild, the purity is high, the cost is relatively low, the operation is simple and convenient, and industrial production is easy.
The invention discloses a preparation method of a phosphodiesterase inhibitor and belongs to the field of medicinal chemistry. The preparation method comprises the following steps: starting the raw material 1 and methanol to prepare the intermediate I without adding organic solvent crystallization. After the series of centrifugation, washing and drying, the intermediate II is directly condensed and cyclized with piperonal, and a final product tadalafil is prepared by chloroacetylation, aminolysis and refining steps. The method improves the product yield and quality, greatly shortens the reaction period, reduces the operation steps and the production cost, and is suitable for industrial mass production.
The invention belongs to the technical field of medicines, and particularly relates to a production process of a tadalafil bulk drug. The production process of the tadalafil bulk drug comprises the following steps: A1, carrying out an esterification reaction on methanol and D-tryptophan to obtain an intermediate I; A2, performing a condensation reaction on the intermediate I and heliotropin to obtain an intermediate II; A3, performing an acylation reaction on the intermediate II and chloroacetyl chloride to obtain an intermediate III; and A4, carrying out a cyclization reaction on the intermediate III and monomethylamine to obtain the tadalafil bulk drug. According to the method, a reaction path is reasonably selected, and meanwhile, the process details of each reaction step are deeply optimized, so high purity and yield of a product of each step of reaction can be obtained, the prepared tadalafil bulk drug is low in cost and good in stability, the economical efficiency of the whole reaction path is improved, and production cost is reduced.
![(6R,12aR)-6-(3,4-dihydroxyphenyl)-2-methyl-2,3,6,7,12,12a-hexahydropyrazino[1',2'-1,6]-pyrido[3,4-b]indole-1,4-dione](/upload/2023/1/b632c696-0d2b-4c5d-8ee6-90470c6ca7ff.png)
(6R,12aR)-6-(3,4-dihydroxyphenyl)-2-methyl-2,3,6,7,12,12a-hexahydropyrazino[1',2'-1,6]-pyrido[3,4-b]indole-1,4-dione

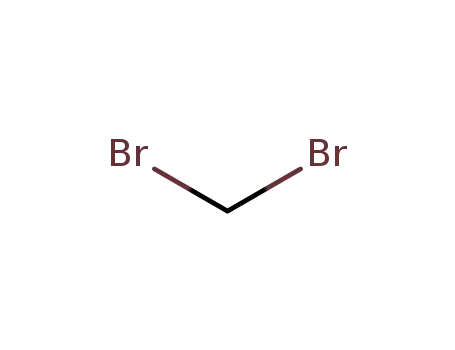
1,1-dibromomethane

![(6R,12aR)-2,3,6,7,12,12a-hexahydro-2-methyl-6-(3,4-methylenedioxyphenyl)-pyrazino[2',1':6,1]pyrido[3,4-b]indole-1,4-dione](/upload/2023/1/14630a06-3880-4f39-8036-2ddcadf18e9c.png)
(6R,12aR)-2,3,6,7,12,12a-hexahydro-2-methyl-6-(3,4-methylenedioxyphenyl)-pyrazino[2',1':6,1]pyrido[3,4-b]indole-1,4-dione
| Conditions | Yield |
|---|---|
|
With sodium hydroxide; In N,N-dimethyl-formamide; at 60 ℃; for 6h;
|
95% |
|
With caesium carbonate; In N,N-dimethyl-formamide; at 80 ℃; for 8h;
|
93% |
|
With caesium carbonate; In N,N-dimethyl-formamide; at 80 ℃; for 8h;
|
93% |
|
With caesium carbonate; In N,N-dimethyl-formamide; at 80 ℃; for 8h;
|
93% |
![(1R,3R)-methyl-1,2,3,4-tetrahydro-2-(2-(benzyl(methyl)amino)acetyl)-1-(3,4-methylenedioxyphenyl)-9H-pyrido[3,4-b]indole-3-carboxylate](/upload/2023/1/db0cef22-6441-4363-a8d8-3714ef3a9ed0.png)
(1R,3R)-methyl-1,2,3,4-tetrahydro-2-(2-(benzyl(methyl)amino)acetyl)-1-(3,4-methylenedioxyphenyl)-9H-pyrido[3,4-b]indole-3-carboxylate

![(6R,12aR)-2,3,6,7,12,12a-hexahydro-2-methyl-6-(3,4-methylenedioxyphenyl)-pyrazino[2',1':6,1]pyrido[3,4-b]indole-1,4-dione](/upload/2023/1/14630a06-3880-4f39-8036-2ddcadf18e9c.png)
(6R,12aR)-2,3,6,7,12,12a-hexahydro-2-methyl-6-(3,4-methylenedioxyphenyl)-pyrazino[2',1':6,1]pyrido[3,4-b]indole-1,4-dione
| Conditions | Yield |
|---|---|
|
With hydrogen; Raney Ni; In ISOPROPYLAMIDE; at 80 ℃; for 22h; under 2280.15 Torr; Product distribution / selectivity;
|
94% |
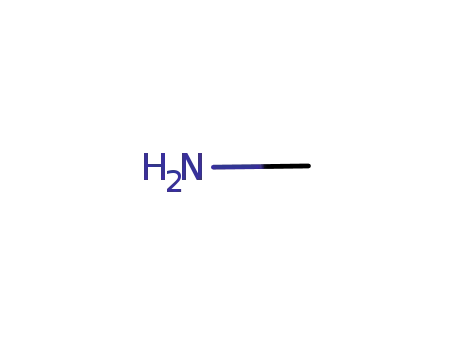
methylamine
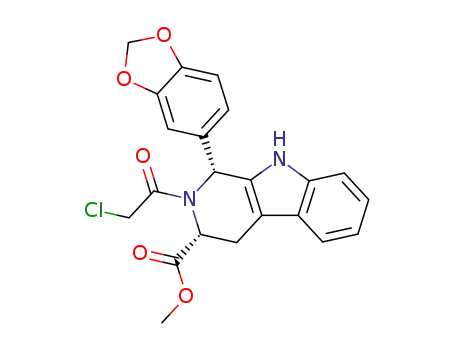
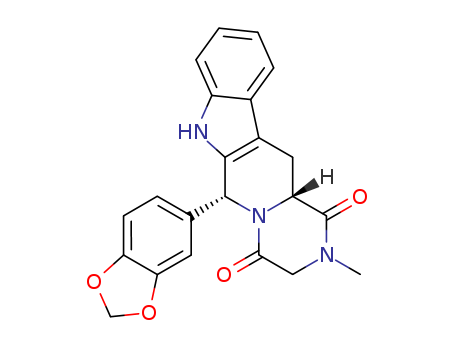
methyl (1R,3R)-1-(3,4-methylenedioxyphenyl)-2-chloroacetyl-2,3,4,9-tetrahydro-9H-pyrido[3,4-b]indol-3-carboxylate
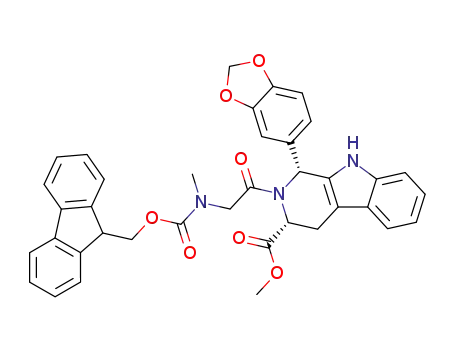
1-benzo[1,3]dioxol-5-yl-2-{[(9H-fluoren-9-ylmethoxycarbonyl)-methyl-amino]-acetyl}-2,3,4,9-tetrahydro-1H-β-carboline-3-carboxylic acid methyl ester
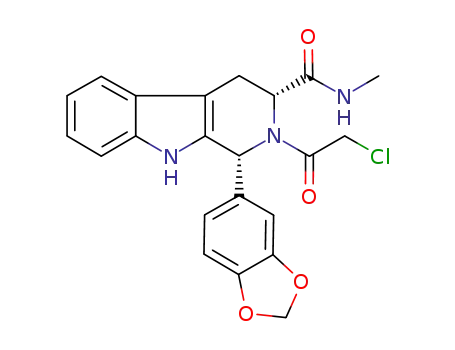
(1R,3R)-1-(benzo[d][1,3]dioxol-5-yl)-2-(2-chloroacetyl)-N-methyl-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxamide
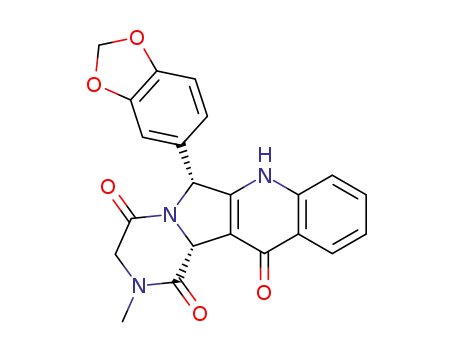
11-benzo[1,3]dioxol-5-yl-3-methyl-2,3,4a,11-tetrahydro-10H-3,10,11a-triaza-benzo[b]fluorene-1,4,5-trione
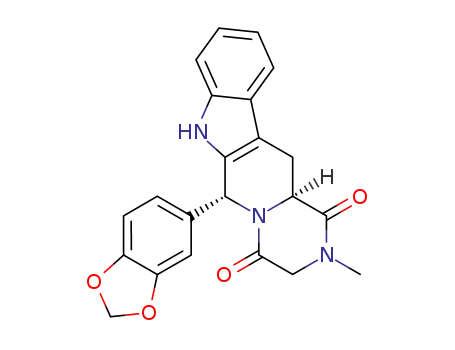
(6R,12aS)-2,3,6,7,12,12a-hexahydro-2-methyl-6-(3,4-methylenedioxyphenyl)-pyrazino[2',1':6,1]pyrido[3,4-b]indole-1,4-dione
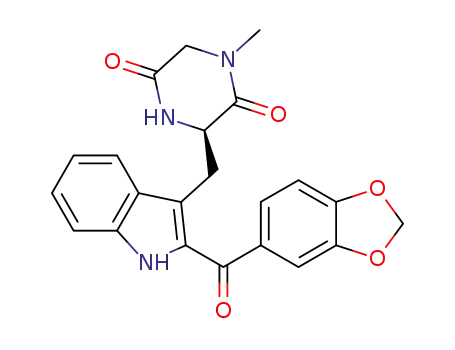
3-((2-(benzo[d][1,3]dioxole-5-carbonyl)-1H-indol-3-yl)methyl)-1-methylpiperazine-2,5-dione
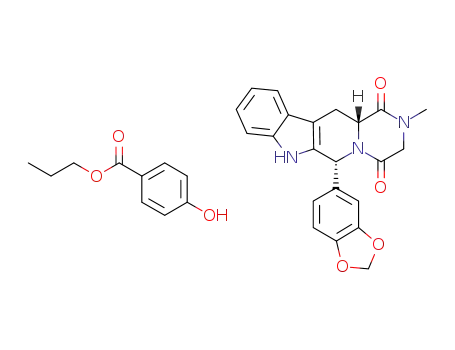
C10H12O3*C22H19N3O4