Your Location:Home > Products > Fine Chemicals > 2-(2-Bromophenyl)-2-propanol
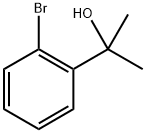

CasNo: 7073-69-0
Molecular Formula: C9H11BrO
|
7073-69-0 Name |
|
|
Name |
2-(2-Bromophenyl)-2-propanol |
|
Synonym |
2-(2-Bromophenyl)-2-propanol;EOS-61013;2-(2-Bromophenyl)propan-2-ol 95+%;Benzenemethanol, 2-bromo-α,α-dimethyl-;Benzenemethanol,2-bromo-a,a-dimethyl-;1-(2-bromophenyl)-1-methylethanol |
|
Chemical & Physical Properties |
|
|
Boiling point |
268.3±15.0 °C at 760 mmHg |
|
Density |
1.4±0.1 g/cm3 |
|
Molecular Formula |
C9H11BrO |
|
Molecular Weight |
215.087 |
|
Flash Point |
116.1±20.4 °C |
|
PSA |
20.23000 |
|
LogP |
2.50 |
|
Exact Mass |
213.999313 |
|
Vapour Pressure |
0.0±0.6 mmHg at 25°C |
|
Index of Refraction |
1.555 |
InChI:InChI=1/C9H11BrO/c1-9(2,11)7-5-3-4-6-8(7)10/h3-6,11H,1-2H3
The invention is applicable to the technical field of medicine synthesis, and provides a synthesis method of a montelukast sodium intermediate. The method comprises the following steps of: generatinga compound II from halogenated benzene under the action
The site-selective modification of polyols bearing several hydroxyl groups without the use of protecting groups remains a significant challenge in synthetic chemistry. Moreover, 1i-catalyzed benzoylation, tosylation, benzylation, and glycosylation of various cis-1,2-diol derivatives proceeded with good yield and site-selective manner.
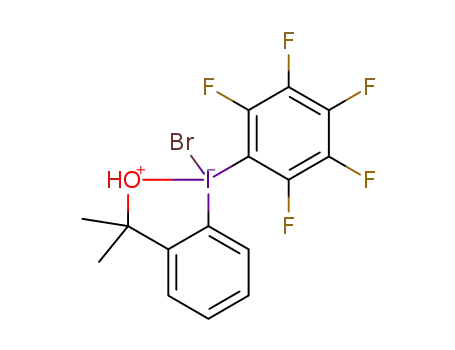
C15H11BrF5IO

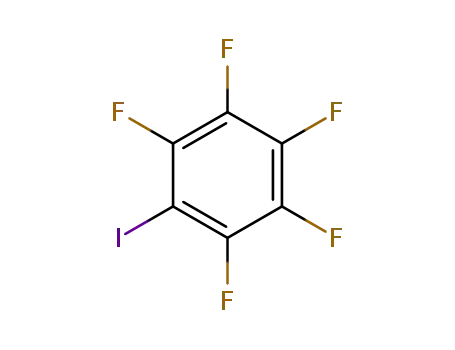
1,2,3,4,5-pentafluoro-6-iodobenzene

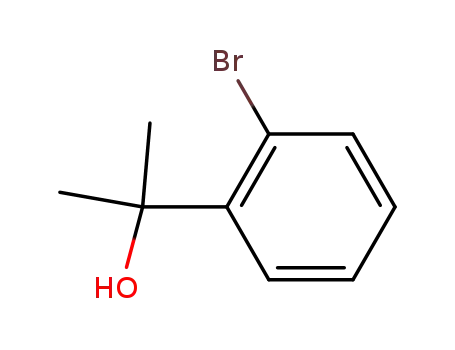
2-(2-bromophenyl)propanol
| Conditions | Yield |
|---|---|
|
In chloroform-d1; at 70 ℃; for 18h;
|
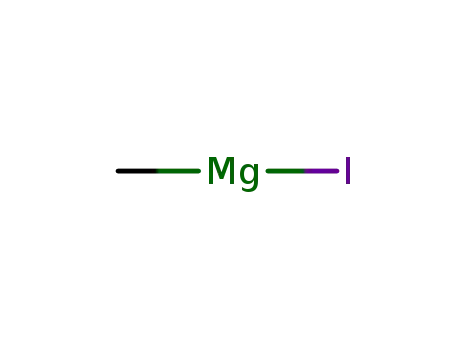
methyl magnesium iodide

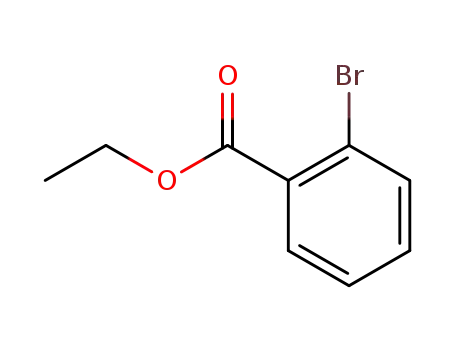
2-bromobenzoic acid ethyl ester


2-(2-bromophenyl)propanol
| Conditions | Yield |
|---|---|
|
In diethyl ether; at 0 - 20 ℃; Inert atmosphere;
|
94% |
|
methyl magnesium iodide; 2-bromobenzoic acid ethyl ester; In diethyl ether;
With ammonium chloride; In diethyl ether; at -5 ℃;
|
71% |
|
With diethyl ether;
|
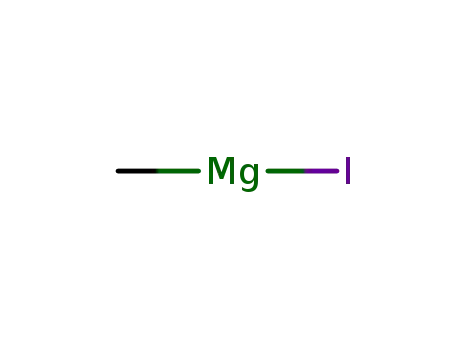
methyl magnesium iodide
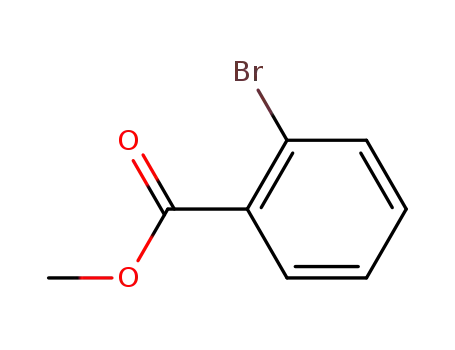
2-bromobenzoic acid methyl ester

2-bromobenzoic acid ethyl ester
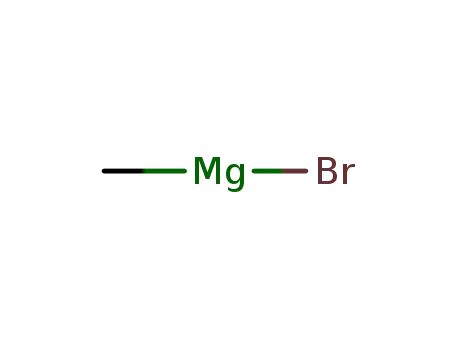
methylmagnesium bromide
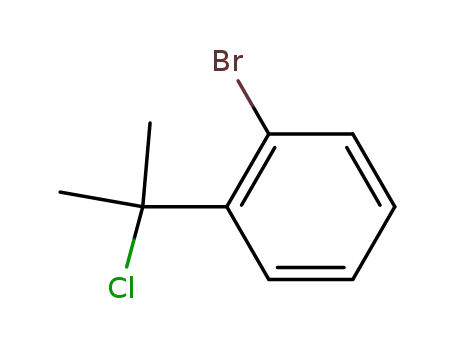
o-Bromo-α,α-dimethylbenzyl chloride
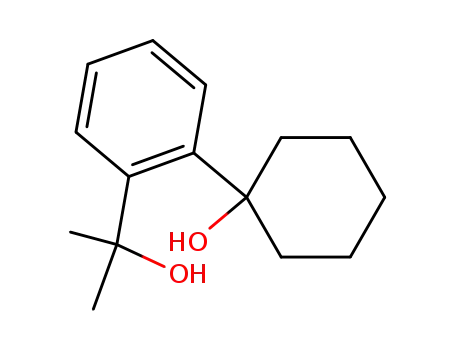
1-[2-(1-hydroxy-1-methylethyl)phenyl]cyclohexanol
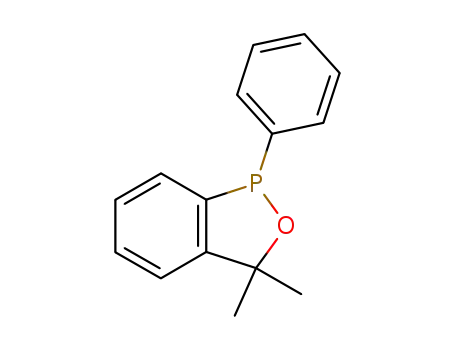
1-phenyl-3,3-dimethyl-3H-2,1-benzoxaphosphole
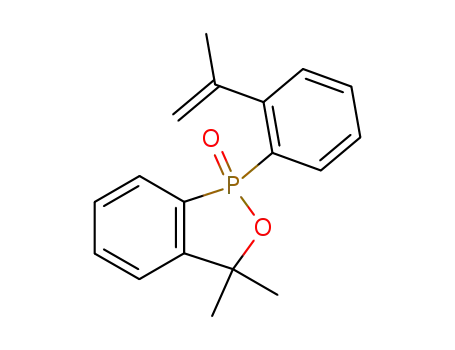
1-(2-isopropenyl-phenyl)-3,3-dimethyl-1,3-dihydro-benzo[c][1,2]oxaphosphole 1-oxide