Your Location:Home > Products > Benzoicacid, 2-bromo-5-chloro-, methyl ester


CasNo: 27007-53-0
Molecular Formula: C8H6BrClO2
Chemical Properties
Colorless liquid
Uses
Methyl 2-bromo-5-chlorobenzoate is used as pharmaceutical intermediate.
InChI:InChI=1/C8H6BrClO2/c1-12-8(11)6-4-5(10)2-3-7(6)9/h2-4H,1H3
The invention provides a 2, 4, 7-trisubstituted fluorene compound and an electronic device thereof. According to the 2, 4, 7-trisubstituted fluorene compound disclosed by the invention, a fluorene rigid structure is introduced, so that the 2, 4, 7-trisubstituted fluorene compound is excellent in film-forming property and thermal stability, and can be used for preparing an organic light-emitting device, an organic field effect transistor and an organic solar cell. In addition, the 2, 4, 7-trisubstituted fluorene compound can be used as a constituent material of a hole injection layer, a hole transport layer, a light emitting layer, an electron blocking layer, a hole blocking layer or an electron transport layer, and can reduce driving voltage, improve efficiency and brightness, prolong theservice life and the like. The preparation method of the 2, 4, 7-trisubstituted fluorene compound is simple, raw materials are easy to obtain, and industrial development requirements can be met.
We report a unique and expeditious route to synthesize 1-isochromanone derivatives through palladium catalyzed tandem Heck coupling/6-endo hydroacyloxylation cyclization between readily available ortho-halogenated benzoates and unactivated alkenes. Various 2-bromo or 2-iodo benzoates can be coupled efficiently with a broad range of alkenes to afford functionalized 1-isochromanones in high yields. Significantly, this cost-efficient and easy-to-handle synthetic methodology will have great prospect application in the synthetic and medicinal chemistry. (Figure presented.).
An operationally simple protocol is disclosed to facilitate entry to benzo-3,4-coumarins directly from biaryl carboxylic acids without the need for substrate prefunctionalization. Complementary to classic lactonization strategies, this disconnection relies on the oxidation competence of photoactivated (-)-riboflavin (vitamin B2) to generate the heterocyclic core via photoinduced single electron transfer. Collectively, the inexpensive nature of the catalyst, ease of execution, and absence of external metal additives are a convincing endorsement for the incorporation of simple vitamins in contemporary catalysis.
The regiochemical outcome of the iodolactonization of 2-alkynylbenzoic acids, carried out at 100 °C in ionic liquids (ILs) as unconventional solvents and with molecular iodine as the iodine source, in the absence of external bases, was found to be strongl
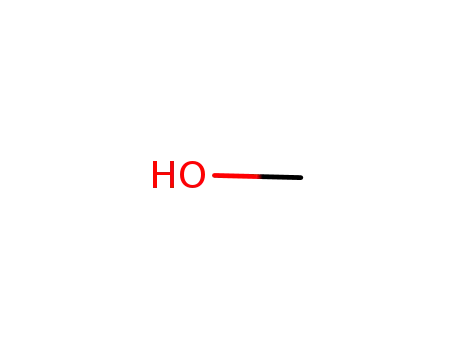
methanol

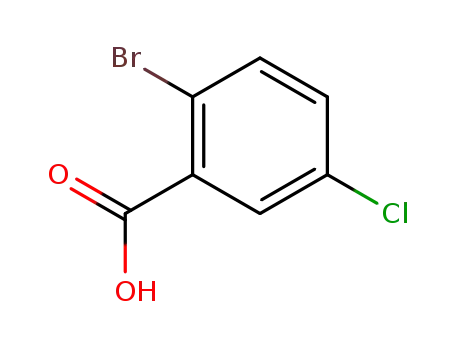
2-bromo-5-chlorobenzoic acid

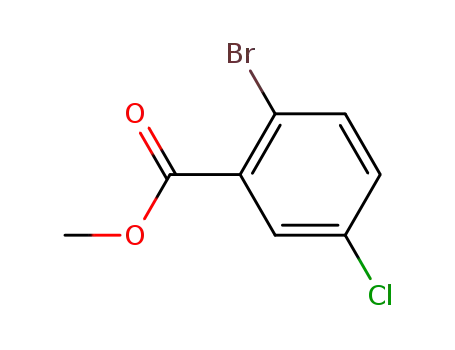
methyl-2-bromo-5-chlorobenzoate
| Conditions | Yield |
|---|---|
|
With acetyl chloride; at 65 ℃; Inert atmosphere;
|
99% |
|
With sulfuric acid; at 65 ℃; for 3h;
|
94% |
|
With hydrogenchloride; at 20 ℃; for 18h;
|
|
|
With sulfuric acid;
|
|
|
With sulfuric acid; for 3h; Reflux;
|
4.17 g |
|
With sulfuric acid; In dichloromethane; water; at 20 ℃; for 3h; Reflux;
|
|
|
With hydrogenchloride; at 20 ℃;
|
|
|
With sulfuric acid; for 3h; Reflux;
|
4.17 g |
|
With sulfuric acid; for 3h; Reflux;
|
4.17 g |
|
With sulfuric acid; for 24h; Reflux;
|
|
|
With sulfuric acid; for 4h; Reflux;
|
|
|
With thionyl chloride; at 80 ℃; Cooling with ice;
|
|
|
With thionyl chloride; at 0 - 20 ℃; for 18h;
|

2-bromo-5-chlorobenzoic acid


methyl-2-bromo-5-chlorobenzoate
| Conditions | Yield |
|---|---|
|
With hydrogenchloride; In methanol;
|
92% |
|
With hydrogenchloride; In methanol;
|
92% |
|
With hydrogenchloride; In methanol;
|
92% |

methanol

2-bromo-5-chlorobenzoic acid
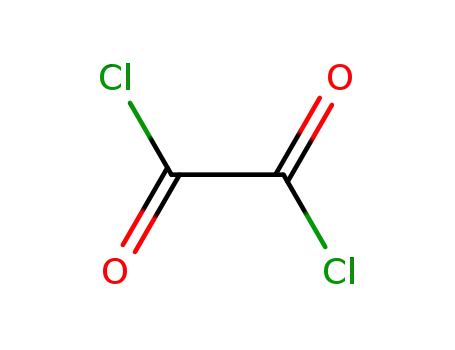
oxalyl dichloride
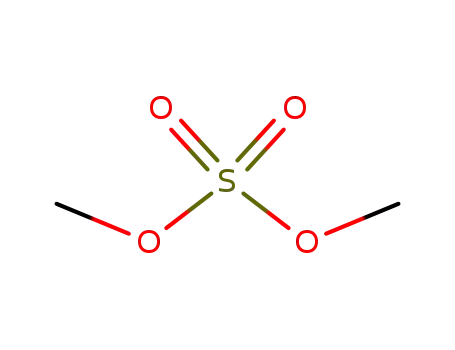
dimethyl sulfate
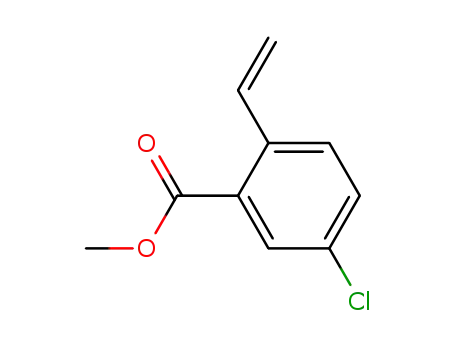
methyl 2-ethenyl-5-chlorobenzoate
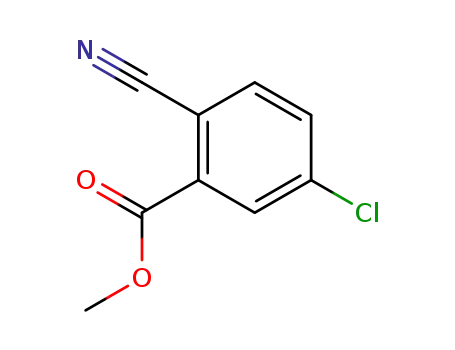
methyl 5-chloro-2-cyanobenzoate
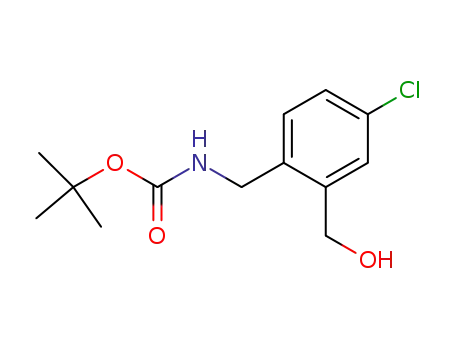
tert-butyl 4-chloro-2-(hydroxymethyl)benzylcarbamate
1-azidomethyl-2-(tert-butyloxycarbonylaminomethyl)-5-chlorobenzene