Your Location:Home > Products > Benzene,1-bromo-4-chloro-2-methyl-


CasNo: 14495-51-3
Molecular Formula: C7H6BrCl
Appearance: Clear colourless to slightly orange liquid
|
14495-51-3 Name |
|
|
Name |
1-Bromo-4-chloro-2-methylbenzene |
|
Synonym |
Benzene,1-bromo-4-chloro-6-methyl-;Toluene, 2-bromo-5-chloro-;5-CHLORO-2-BROMOTOLUENE;6-BROMO-3-CHLOROTOLUENE;2-BROMO-5-CHLOROTOLUENE;1-BROMO-4-CHLORO-2-METHYLBENZENE;2-BROMO-5-CHLOROTOLUENE 99%;2-Bromo-5-Chlorotolune98% |
|
14495-51-3 Chemical & Physical Properties |
|
|
Boiling point |
219.1±20.0 °C at 760 mmHg |
|
Density |
1.5±0.1 g/cm3 |
|
Molecular Formula |
C7H6BrCl |
|
Molecular Weight |
205.480 |
|
Flash Point |
95.6±0.0 °C |
|
LogP |
3.98 |
|
Exact Mass |
203.934128 |
|
Vapour Pressure |
0.2±0.4 mmHg at 25°C |
|
Index of Refraction |
1.566 |
|
Water Solubility |
INSOLUBLE |
1-Bromo-4-chloro-2-methylbenzene 14495-51-3 is CLEAR COLOURLESS TO SLIGHTLY ORANGE LIQUID. It has been used in the preparation of 4-chloro-2-methylbenzophenone.
InChI:InChI=1/C7H6BrCl/c1-5-4-6(9)2-3-7(5)8/h2-4H,1H3
Excellent conversion rates, product yields, and selectivities were also found for other halides, for example, 1-bromo-4-chloro-2-methylbenzene, 1-bromo-2,6-dichlorobenzene…Dichloro-bis(aminophosphine) complexes are stable depot forms of palladium nanoparticles and have proved to be excellent Suzuki–Miyaura catalysts. Simple modifications of the ligand (and/or the addition of water to the reaction mixture) have allowed their formation to be controlled.
Highly effective embodiment of Sandmeyer reaction has been revealed for Cu-based catalysts incorporating ionic liquid on Silochrom support. The most active catalyst (TOF = = 4000–8000 h–1) contains comparable amounts of cuprous and cupric chloride anions. The reported method allows one to carry out the reaction for anilines in the one-pot mode.
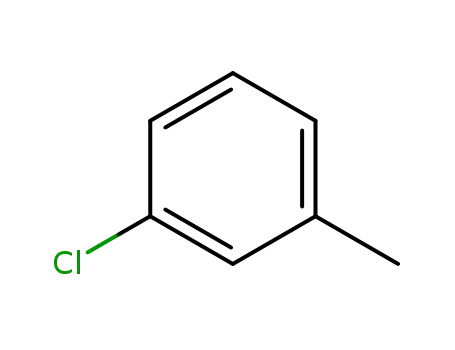
1-chloro-3-methylbenzene

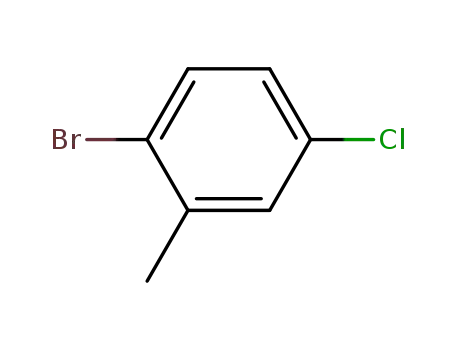
2-bromo-5-chlorotoluene

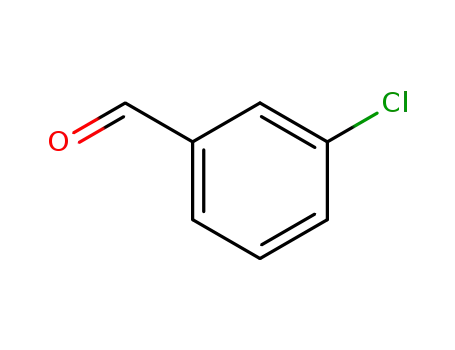
m-Chlorobenzaldehyde
| Conditions | Yield |
|---|---|
|
With potassium permanganate; alkyl carboxylic acid; corresponding anhydride; potassium bromide; Yield given. Yields of byproduct given; Heating;
|
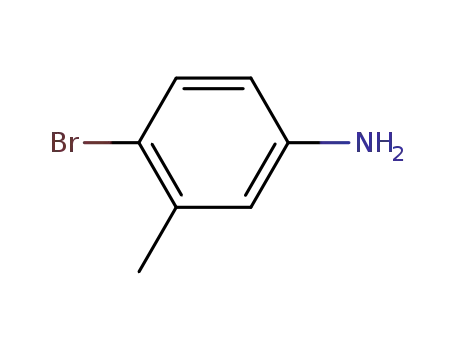
amino-5-bromo-2-toluene


2-bromo-5-chlorotoluene
| Conditions | Yield |
|---|---|
|
amino-5-bromo-2-toluene; With boron trifluoride diethyl etherate; tetraethylammonium chloride; In acetonitrile; at 0 ℃; for 0.166667h;
With tert.-butylnitrite; In acetonitrile; at 0 - 20 ℃; for 1h;
|
90% |

1-chloro-3-methylbenzene
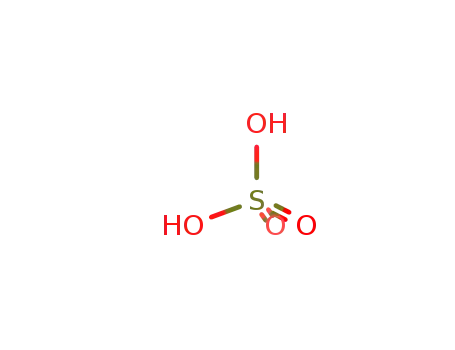
sulfuric acid
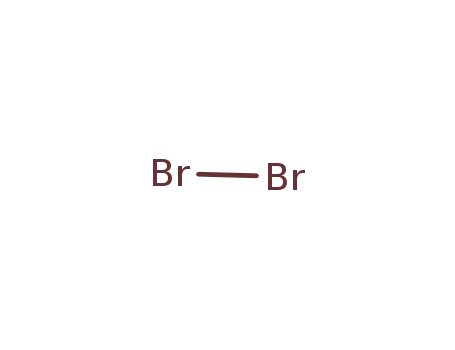
bromine
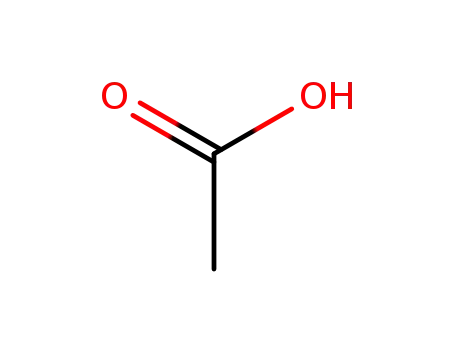
acetic acid
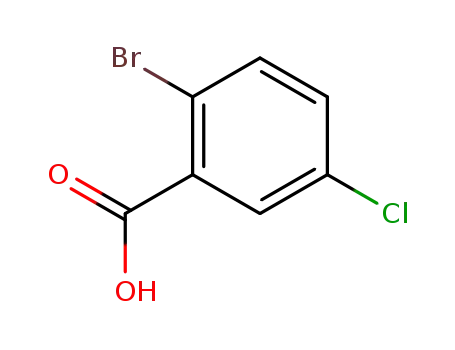
2-bromo-5-chlorobenzoic acid
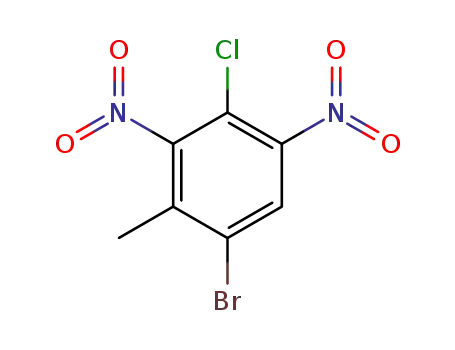
1-bromo-4-chloro-2-methyl-3,5-dinitro-benzene
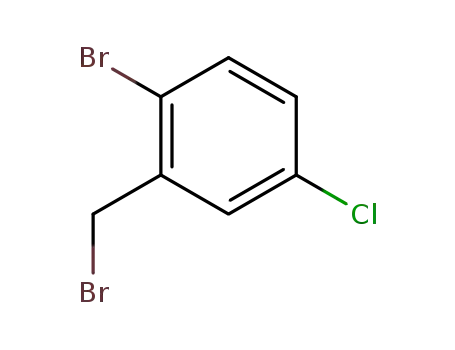
2-bromo-5-chlorobenzyl bromide
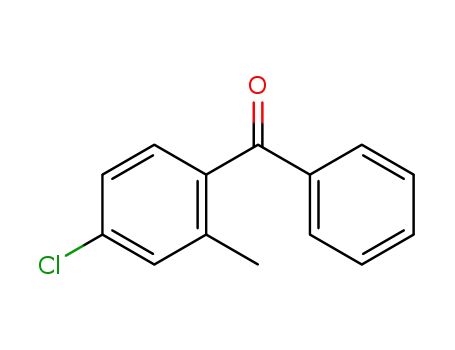
(4-chloro-2-methylphenyl)(phenyl)methanone