Your Location:Home > Products > Pregabalin
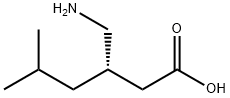

CasNo: 148553-50-8
Molecular Formula: C8H17NO2
Appearance: off-white solid
Description
Pregabalin is a second- generation antiepileptic drug (AED) known with the proprietary brand name of Lyrica? (Pfizer, Tadworth) in the UK and USA (Pfizer, New York, NY).
Generic formulation
MHRA/ CHM advice to minimize risk when switching patients with epilepsy between different manufacturers’ products (including generic products): It is usually unnecessary to ensure that patients are maintained on a specific manufacturer’s product unless there are specific concerns, such as patient anxiety and risk of confusion/ dosing error.
Indications
Epilepsy Adjunctive therapy of focal seizures with and without secondary generalization. Recommendations summarized from NICE (2012) Seizure types—on referral to tertiary care (focal seizures), contraindicated (generalized tonic- clonic seizures, tonic/ atonic seizures, absence seizures, myoclonic seizures). Epilepsy types—on referral to tertiary care (benign epilepsy with centrotemporal spikes, panayiotopoulos syndrome, late- onset childhood occipital epilepsy), contraindicated (absence syndromes, idiopathic generalized epilepsy, juvenile myoclonic epilepsy, Dravet syndrome, Lennox– Gastaut syndrome). Psychiatry Generalized anxiety disorder. Neurology Peripheral and central neuropathic pain.
Dose titration
Epilepsy— adjunctive therapy: 25 mg bd for 7 days, to be increased by 50 mg every 7days; usual maintenance 300 mg daily, divided into 2 or 3 doses (max. 600 mg daily, divided into 2 or 3 doses). Generalized anxiety disorder: 150 mg daily, divided into 2 or 3 doses, for 7 days, to be increased by 150 mg every 7 days (max. 600 mg daily, divided into 2 or 3 doses). If stopping pregabalin, it is recommended to taper over at least 1?week to avoid abrupt withdrawal.
Plasma levels monitoring
Pregabalin pharmacokinetics are linear over the recommended daily dose range; inter- subject pharmacokinetic variability for pregabalin is low (<20%) and multiple dose pharmacokinetics are predictable from single- dose data. Therefore, there is no need for routine monitoring of plasma concentrations of pregabalin.
Antiepileptic drus and therapeutic drugs for neuropathic pain
Pregabalin is a new antiepileptic drug, having a γ-amino butyric acid structure on its molecular structure, which has anticonvulsant effects, and is successfully developed by the company Pfizer for the treatment of peripheral neuropathic pain, or adjuvant treatment of partial seizures. In December 2008, the US Food and Drug Administration (FDA) approved pregabalin (trade name "Lyrica") for the treatment of diabetic peripheral neuropathic pain (DPN) and postherpetic neuralgia (PHN)which are Both the most common neuropathic pains. Neuropathic pain is one of the most difficult chronic pain syndromes to treat , dull pain, burning, tingling as the main feature, there are a lot of incentives of neuralgia, diabetes, infections (such as herpes zoster), cancer and AIDS, etc. can cause neurological pain, in Europe about 3% of the population suffer from neuralgia torture.
Cautions
Patients with conditions that may precipitate encephalopathy. Patients with severe congestive heart failure.
Adverse effects
Pregabalin can be associated with adverse effects at the level of the nervous system and other systems.
Interactions
Since pregabalin is predominantly excreted unchanged in the urine, undergoes negligible metabolism in humans, does not inhibit drug metabolism in vitro, and is not bound to plasma proteins, it is unlikely to produce or be subject to pharmacokinetic interactions. Pregabalin may potentiate the effects of lorazepam. In the post- marketing experience, there are reports of respiratory failure and coma in patients taking pregabalin and other central nervous system depressant medicinal products. Pregabalin appears to be additive in the impairment of cognitive and gross motor function caused by oxycodone. With alcohol/food There are no specific foods that must be excluded from diet when taking pregabalin. Pregabalin may potentiate the effects of alcohol.
Special populations
Hepatic impairment No dose adjustment is required for patients with hepatic impairment. Renal impairment Reduce maintenance dose according to degree of reduction in creatinine clearance. Pregnancy There is no adequate data from the use of pregabalin in pregnant women. The potential risk for reproductive toxicity in humans is unknown. Pregabalin should not be used during pregnancy unless the benefit to the mother clearly outweighs the potential risk to the foetus. Pregabalin is excreted into human milk. The effect of pregabalin on newborns/ infants is unknown. A case- by- case decision must be made whether to discontinue breast- feeding or to discontinue pregabalin therapy taking into account the benefit of breastfeeding for the child and the benefit of therapy for the woman.
Behavioural and cognitive effects in patients with epilepsy
Pregalin is characterized by a good behavioural profile. This AED does not appear to have significant negative effects on mood or behaviour in patients with epilepsy, although depression has been reported in some patients (dose- dependent effects of mild- to- moderate intensity). A potential abuse or misuse of pregabalin has also been reported, with implications in terms of dependence and withdrawal. pregabalin is also associated with limited negative cognitive effects, mainly related to sedation, decreased arousal, decreased attention and concentration (dose- dependent effects of mild- to- moderate intensity).
Psychiatric use
Pregabalin has an approved indication and is widely used for the treatment of generalized anxiety disorder. Several randomized, double- blind, placebocontrolled trials found that pregabalin is an effective treatment for patients with generalized anxiety disorder and social anxiety disorder. Possible implications in the treatment of mood disorders and benzodiazepines dependence are emerging. Moreover, pregabalin may be a therapeutic agent for the treatment of alcohol abuse, in both withdrawal phase and relapse prevention.
Description
Pregabalin (Lyrica), an analog of GABA that is related to gabapentin, has been extensively studied in the treatment of GAD, fibromyalgia, neuropathic pain, and partial complex seizures.It appears to act even more selectively on the a2-δ subunit of calcium channels found in brain than does gabapentin. Pregabalin received FDA approval in late 2004 for use in treating neuropathic pain and epilepsy. In 2008, it became the first drug approved for the treatment of fibromyalgia.Even though prcgabalin reccived approval for the treatment of GAD in the European Union in 2006, the FDA has not approvedpregabalin for GAD in the United States as of this writing.With the consistent efficacy in clinical trials and the known safety of pregabalin in clinical expericnce, it is not completely clear what has held up approval of prcgabalin for GAD.There was reported concern of the risk-benefit ratio for the drug,given the hepatotoxicity seen in micc, but this has not proven to be a problem postrelease in humans. Pregabalin is categorized as an anticonvulsant and analgesic. The drug is under re-review currently by the FDA. Pregabalin has a potential for abuse and misuse. Pregabalin is regulated as a Schedule V compound in the United States.
Chemical Properties
Off-White Solid
Originator
Warner-Lambert (US)
Uses
S-Enantiomer of Pregabalin. A GABA analogue used as an anticonvulsant. Anxiolytic analgesic used to treat peripheral neuropathic pain and fibromyalgia.
Uses
Pregabalin is an anticonvulsant drug used for neuropathic pain, as an adjunct therapy for partial seizures, and in generalized anxiety disorder. It was designed as a more potent successor to gabapentin. Pregabalin is marketed by Pfizer under the trade nam
Brand name
Lyrica (CP).
General Description
Pregabalin, marketed as the anticonvulsant drug Lyrica, is used in the treatment of epilepsy and generalized anxiety disorder. Along with other anticonvulsants such as gabapentin and levetiracetam, pregabalin is also used to treat neuropathic pain. This certified reference standard is suitable for pregabalin GC/MS or LC/MS applications from forensic analysis, clinical toxicology and pain prescription monitoring to urine drug testing.
Biochem/physiol Actions
Pregabalin is a lipophilic GABA analog/ligand at α2δ subunit of voltage-dependent Ca2+ channels. Pregabalin is an anticonvulsant, anxiolytic analgesic used to treat peripheral neuropathic pain and fibromyalgia.
Clinical Use
Antiepileptic Neuropathic pain Generalised anxiety disorder
Synthesis
Several syntheses of pregabalin (X) have been disclosed in the literature, including process scale-up comparison of several different routes. The most cost efficient route as described in the publication is shown in the Scheme. Condensation of diethyl malonate 69 in the presense of diisopropyl amine in acetic acid gave a,b-unsaturated diester 70 in high yield. Reaction of the enone diester with potassium cyanide gave cyano diester 71 in 95% yield. In a remarkable three step, one pot process, the nitrile in 71 was hydrolyzed followed by decarboxylation of one of the esters to provide 72 in 73% yield. Resolution of the two enantiomers was achieved using (S)-(+)-mandellic acid, one of the best acid found after many salt screening, to give, after two recrystallization, a 99:1 ratio of the desired diastereomer.Removal of the acid was done with wet THF instead of base separation, to avoid salt impurities, and one recrystallization in ethanol gave 100% ee diastereomer in 25 – 29% overall yield.It’s worth noting that the Pfizer group have come up with a new process of preparing pregabalin (X) via enantioselective reduction, that promises to further reduce cost and waste associated with the manufacture of this drug.
Drug interactions
Potentially hazardous interactions with other drugs Antidepressants: anticonvulsant effect antagonised. Antimalarials: anticonvulsant effect antagonised by mefloquine. Antipsychotics: anticonvulsant effect antagonised. Orlistat: possible increased risk of convulsions.
Metabolism
Pregabalin undergoes negligible metabolism, and about 98% of a dose is excreted in the urine as unchanged drug.
InChI:InChI=1/C8H17NO2/c1-6(2)3-7(5-9)4-8(10)11/h6-7H,3-5,9H2,1-2H3,(H,10,11)/t7-/m0/s1
The chromatographic resolution of pregabalin enantiomers has been often achieved by derivatization of the molecule, in order to reach enough sensitivity at low concentrations of the minor enantiomer present in the active principle. In the present article, the development and optimization of two liquid chromatographic methods are presented for the direct resolution of pregabalin enantiomers on a chiral stationary phase (CSP) containing a zwitterionic selector derived from cinchona alkaloid and sulfonic acid (CHIRALPAK ZWIX). The key parameters for the separation as well as the compatibility of chromatographic conditions with different detection modes (ultraviolet and mass spectrometry) were investigated. The resulting methods were found to be selective, of high performance and low limits of detection (2 μg/mL by UV and 1 ng/mL by MS, respectively) and quantification (6 μg/mL by UV and 5 ng/mL by MS, respectively) for the minor enantiomer which is considered as a chiral impurity.
A simple and cost-effective process for racemization of undesired (S)-3-(carbamoylmethyl)-5-methylhexanoic acid (9), produced during the resolution step, is described. The literature procedure is fraught with many difficulties including number of steps and hazardous reagents. We have developed a one pot process for the above-mentioned racemization of S-enantiomer. The basic objective is to convert S-enantiomer into the symmetrical glutarimide derivative followed by hydrolysis with an alkali. The transformation of 9 into glutarimide derivative (10) has been achieved with piperidine in refluxing toluene.
An efficient chemoenzymatic route has been developed for the synthesis of optically active pregabalin (PGB) from isobutylsuccinonitrile (IBSN). (S)-3-cyano-5-methylhexanoic acid ((S)-CMHA), a critical chiral intermediate of PGB, was synthesized using regio- and enantioselective hydrolysis of IBSN by immobilized Escherichia coli cells harboring nitrilase BrNIT from Brassica rapa. The catalytic performances of immobilized cells were investigated, and high enantioselectivity (E > 150) and substrate conversion (>41.1%) were obtained at a substrate loading of 100 g/L by immobilized cells after 12 batches of reaction. The unreacted (R)-IBSN was recycled by racemization with a high yield of 94.5%, and the resultant (S)-CMHA was hydrogenated directly to the desired PGB with a high purity of 99.6% and optical purity of 99.4%. The input of raw materials and E factor of this chemoenzymatic route were demonstrated to be much lower than those of the first- and second-generation routes for PGB synthesis.
Asymmetric bioreduction of an (E)-β-cyano-2,4-dienoic acid derivative by ene-reductases allowed a shortened access to a precursor of pregabalin [(S)-3-(aminomethyl)-5-methylhexanoic acid] possessing the desired configuration in up to 94% conversion and >99% ee. Deuterium labelling studies showed that the nitrile moiety was the preferred activating/anchor group in the active site of the enzyme over the carboxylic acid or the corresponding methyl ester.
We reported the synthesis of (S)-pregabalin in a five-step sequence with 20% overall yield. As a modification of the previously developed route, a Michael addition between CH3NO2 and chiral oxaoxazolidinone was employed as a key operation for introducing the methyleneamino group, which allowed avoiding the use of toxic cyanide reagent and led to enantiomerically pure product (>99% ee) after the recrystallization in appropriate solvent.
Desymmetrization of prochiral 3-substituted glutaronitriles offers a new approach to access (S)-Pregabalin and (R)-Baclofen. A number of nitrilases from diverse sources were screened with 3-isobutylglutaronitriles (1a) or 3-(4′-chlorophenyl)glutaronitriles (1b) as the substrate. Some nitrilases were found to catalyze the desymmetric hydrolysis of 1a and 1b to form optically active 3-(cyanomethyl)-5-methylhexanoic acid (2a) and 3-(4′-chlorophenyl) -4-cyanobutanoic acid (2b) with high enantiomeric excesse (ee), respectively. This cannot be achieved using traditional chemical hydrolysis. Among them, AtNIT3 generated (R)-2b, whereas BjNIT6402 and HsNIT produced the opposite (S)-enantiomer with high conversions and ee values. Not only the nitrilases showed different activities and stereoselectivities toward these 3-substituted glutaronitriles, the 3-substituent of the substrates also exerted great effect on the enzyme activity and stereoselectivity. (S)-2a and (S)-2b were prepared with high yields and ee values using BjNIT6402 and HsNIT as the biocatalysts, respectively. A straightforward Curtius rearrangement of (S)-2a and (S)-2b, followed by the acidic hydrolysis, afforded (S)-Pregabalin and (R)-Baclofen. This offers a new platform methodology for the synthesis of optically active β-substituted γ-amino acids of pharmaceutical importance.
(Chemical Equation Presented) Chiral β-substituted γ-butyrolactones are known to be important intermediates for many biologically active compounds such as γ-aminobutyric acid (GABA) derivatives and lignans. We have developed a general, convenient, and scalable synthetic method for enantiomerically pure β-substituted γ-butyrolactones, with either configuration, via nucleophilic cyclopropane ring opening of (1S,5R)- or (1R,5S)-bicyclic lactone followed by decarbethoxylation. The utility of our method was demonstrated by streamlined synthesis of pregabalin ((S)-3-isobutyl-γ-aminobutyric acid), an anticonvulsant drug for the treatment of peripheral neuropathic pain.
A new manufacturing process for (S)-3-(aminomethyl)-5-methylhexanoic acid (Pregabalin), the active ingredient in Lyrica, has been developed. Using Lipolase, a commercially available lipase, rac-2-earboxyethyl-3-cyano-5- methylhexanoic acid ethyl ester (1) can be resolved to form 2-carboxyethyl-3- cyano-5-methylhexanoic acid (2). A heat-promoted decarboxylation of 2 efficiently generates (S)-3-cyano-5-rnethylhexanoic acid ethyl ester (3), a known precursor of Pregabalin. This new route dramatically improved process efficiency compared to the first-generation process by setting the stereocenter early in the synthesis and enabling the facile racemization and reuse of (R)-l. The chemoenzymatic process also reduced organic solvent usage resulting in a mostly aqueous process. Compared to the first-generation manufacturing process, the new process resulted in higher yields of pregabalin (40-45% after one recycle of (R)-l), and substantial reductions of waste streams corresponding to a 5-fold decrease in the E factor from 86 to 17.
A concise enantioselective synthesis of (S)-(+)-3-aminomethyl-5-methylhexanoic acid (1, Pregabalin) has been developed. The key step is the asymmetric hydrogenation of a 3-cyano-5-methylhex-3-enoic acid salt 2 with a rhodium Me-DuPHOS catalyst, providing the desired (S)-3-cyano-5-methylhexanoate 3 in very high ee. Subsequent hydrogenation of the nitrile 3 with a heterogeneous nickel catalyst provides Pregabalin I in excellent overall yield and purity.
A straightforward synthesis of (S)-pregabalin in 28% overall yield starting from d-mannitol acetonide, as a primary source of chirality, is presented. The process is suitable for large-scale synthesis and involves simple and high-yielding chemical transformations as well as low-cost commercially available reagents.
The present invention relates to a process for the preparation of gamma aminobutyric acid derivatives of formula I, in particular pregabalin, baclofen and analogs thereof. Further, this process is comprised of preparation protocol for compounds of formula I, involving Michael addition and Beckmann rearrangement strategy.
The present invention relates to a method for preparing pregabalin by a biological enzyme method. In particular, the method comprises producing pregabalin B and an R-configuration compound C by using a compound A as a raw material under the action of a biological enzyme; performing configuration inversion of the separated and recovered R-configuration compound C under the action of an isomerase to produce an S-configuration compound D; and producing pregabalin B from the compound D under the action of a biological enzyme
In one aspect the invention relates to a method of treatment selected from the group consisting of: (a) treating a symptom such as pain in a subject identified or diagnosed as having Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS); (b) treating a symptom such as pain in a subject having dysfunctional TRPM3 ion channel activity; (c) restoring NK cell function in a subject having dysfunctional TRPM3 ion channel activity; and (d) restoring calcium homeostasis in a subject having dysfunctional TRPM3 ion channel activity. The method comprises the step of administering to the subject a therapeutically effective amount of at least one therapeutic compound selected from the group consisting of: (i) an opioid receptor antagonist; (ii) an opioid antagonist; and (iii) a therapeutic compound that restores TRPM3 ion channel activity. In some embodiments the therapeutic compound is naltrexone hydrochloride.
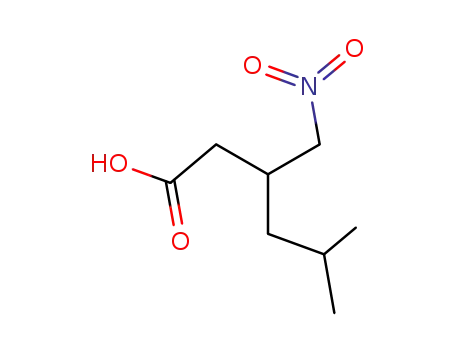
5-methyl-3-(nitromethyl)hexanoic acid

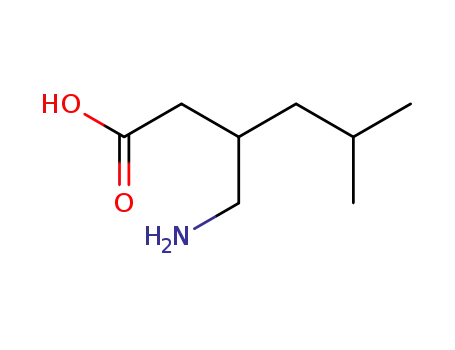
(R,S)-3-iso-butyl-4-aminobutyric acid
| Conditions | Yield |
|---|---|
|
With palladium 10% on activated carbon; hydrogen; In methanol; at 25 ℃; for 24h; under 760.051 Torr;
|
97% |
|
With hydrogen; 5%-palladium/activated carbon; In methanol; at 25 - 30 ℃; for 6 - 8h;
|
80% |
|
With hydrogen; 5%-palladium/activated carbon; In methanol; for 5 - 8h;
|
37% |
|
With hydrogen; 5% Pd(II)/C(eggshell); In methanol;
|
37% |
|
With hydrogen; nickel; In methanol; for 12h; under 2942.29 Torr;
|
|
|
With palladium 10% on activated carbon; hydrogen; In methanol; at 60 ℃; under 45004.5 Torr;
|
630 mg |
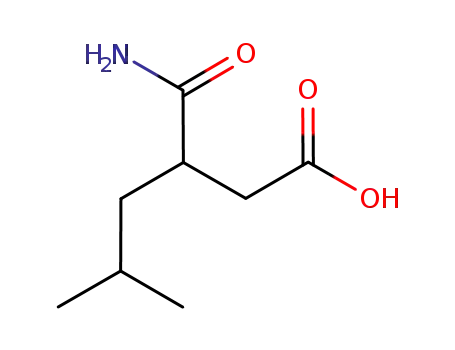
3-carbamoyl-5-methylhexanoic acid


(R,S)-3-iso-butyl-4-aminobutyric acid
| Conditions | Yield |
|---|---|
|
With lithium borohydride; In tetrahydrofuran; methanol; at 10 ℃; Reagent/catalyst;
|
88.4% |
|
With sodium hydroxide; In water; at 0 - 10 ℃;
|
79.8% |
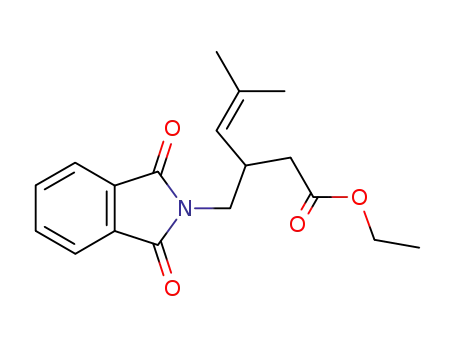
3-(1,3-Dioxo-1,3-dihydro-isoindol-2-ylmethyl)-5-methyl-hex-4-enoic acid ethyl ester
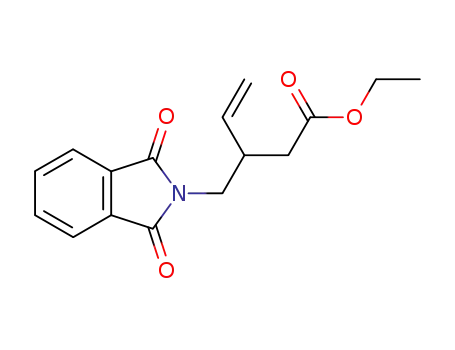
3-(1,3-Dioxo-1,3-dihydro-isoindol-2-ylmethyl)-pent-4-enoic acid ethyl ester
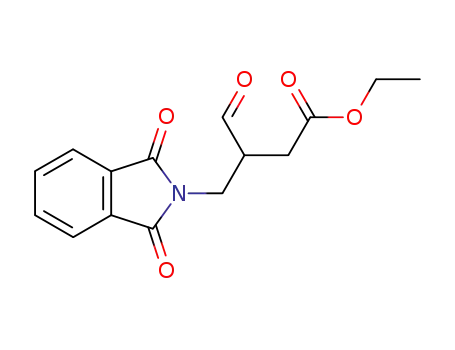
4-(1,3-Dioxo-1,3-dihydro-isoindol-2-yl)-3-formyl-butyric acid ethyl ester
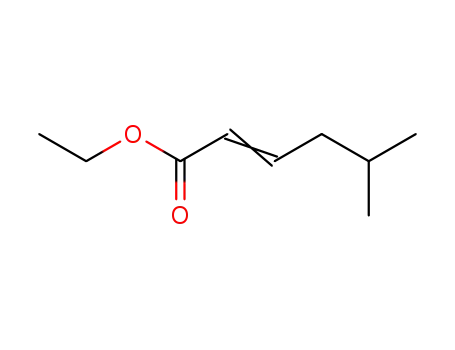
5-methyl-2-hexenoic acid ethyl ester
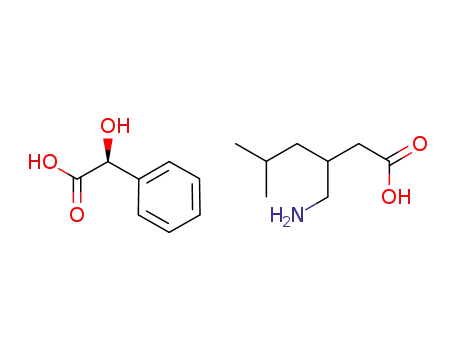
C8H8O3*C8H17NO2
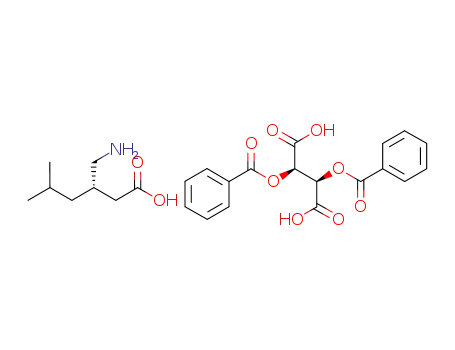
(S)-pregabalin-(-)-O,O'-dibenzoyl-L-tartrate
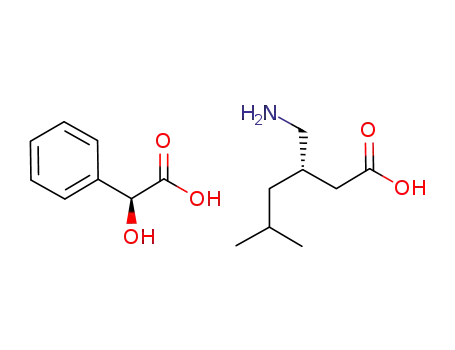
(S)-(+)-3-(aminomethyl)-5-methylhexanoic acid (S)-mandelate
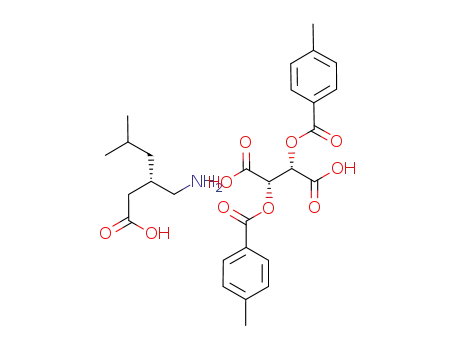
(S)-(+)-3-aminomethyl-5-methylhexanoic acid di-(O,O')-p-toluoyl-D-tartaric acid salt