Your Location:Home > Products > Fine Chemicals > Memantine HCL


CasNo: 41100-52-1
Molecular Formula: C12H21N.HCl
Appearance: Crystalline solid
|
41100-52-1 Name |
|
|
Name |
Memantine HCL |
|
Synonym |
MEMANTINE HCL;LABOTEST-BB LT00233220;1,3-Dimethyl-5-aminoadamantane hydrochloride;1,3-DIMETHYLAMINOADAMANTANE HYDROCHLORIDE;1-AMINO-3,5-DIMETHYLADAMANTANE HCL;1-AMINO-3,5-DIMETHYLADAMANTANE HYDROCHLORIDE;1-AMINO-3,4-DIMETHYLADAMANTANE HYDROCHLORIDE;3,5-DIMETHYLAMANTADINE HYDROCHLORIDE |
|
41100-52-1 Biological Activity |
|
|
Related Catalog |
Signaling Pathways >> Autophagy >> Autophagy Signaling Pathways >> Metabolic Enzyme/Protease >> Cytochrome P450 Signaling Pathways >> Membrane Transporter/Ion Channel >> iGluR Signaling Pathways >> Neuronal Signaling >> iGluR Research Areas >> Neurological Disease |
|
41100-52-1 Chemical & Physical Properties |
|
|
Melting point |
292 °C |
|
Boiling point |
239.8ºC at 760 mmHg |
|
Molecular Formula |
C12H22ClN |
|
Molecular Weight |
215.763 |
|
Flash Point |
92.3ºC |
|
PSA |
26.02000 |
|
LogP |
4.19640 |
|
Exact Mass |
215.144073 |
|
Storage condition |
-20°C Freezer |
Memantine HCl is the HCl form of Memantine. Memantine hydrochloride, also often known to as 1-amino-3,5-dimethyl-adamantane hydrochloride , is a novel drug used to treat Alzheimer's disease. Memantine hydrochloride (Namenda; Forest/Merz), which acts to protect neurons against toxicity caused by overactivation of N-methyl-D-aspartate receptors. Memantine HCl (ME-HCl) is a novel class of Alzheimer’s disease medication acting on glutamatergic system by blocking NMDA glutamate receptors.
InChI:InChI=1/C12H21N.ClH/c1-10-3-9-4-11(2,6-10)8-12(13,5-9)7-10;/h9H,3-8,13H2,1-2H3;1H
The density (ρ), speed of sound (u), and refractive index (nD) of aqueous solutions of Memantine hydrochloride with molalities ranging from 0.0 to 0.05 at atmospheric pressure and temperatures between 305.15 and 320.15 K were investigated. A thorough review of the literature indicates a paucity of data on volumetric properties of aqueous solutions of memantine hydrochloride. Consequently, we intended on conducting investigations on memantine hydrochloride in water that included volumetric, optical, and molecular dynamic studies at different temperatures.
Modern photoredox catalysis has traditionally relied upon metal-to-ligand charge-transfer (MLCT) excitation of metal polypyridyl complexes for the utilization of light energy for the activation of organic substrates. Furthermore, the LMCT excitation event has been investigated through a series of spectroscopic experiments, revealing a rapid bond homolysis process and an effective production of alkoxy radicals, collectively ruling out the LMCT/homolysis event as the rate-determining step of this C-H functionalization.
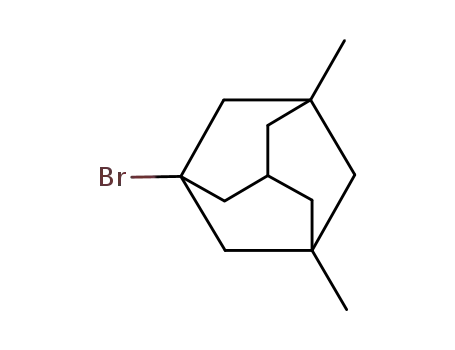
3,5-dimethyl-1-bromoadamantane

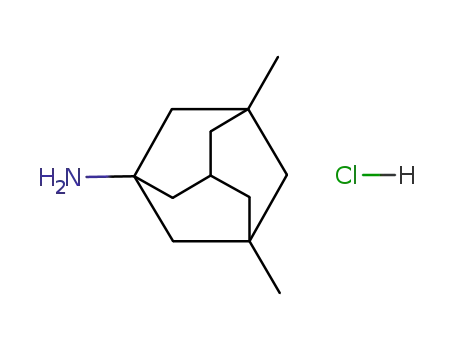
memantine hydrochloride
| Conditions | Yield |
|---|---|
|
3,5-dimethyl-1-bromoadamantane; With sulfuric acid; toluene-4-sulfonic acid; acetonitrile; at 30 - 50 ℃; for 2h;
With hydrogenchloride; In water; ethyl acetate; for 2h; Reagent/catalyst;
|
88.44% |
|
3,5-dimethyl-1-bromoadamantane; With formic acid; urethane; at 20 - 90 ℃; for 1h; Inert atmosphere;
With hydrogenchloride; In water; at 20 - 110 ℃; for 4h; Reagent/catalyst; Temperature; Inert atmosphere;
|
85% |
|
Multi-step reaction with 3 steps
1: sulfuric acid / 12 h
2: sodium hydroxide / water; ethylene glycol / 12 h / 150 °C
3: hydrogenchloride / pH 6 / Gas phase
With hydrogenchloride; sulfuric acid; sodium hydroxide; In water; ethylene glycol;
|
|
|
Multi-step reaction with 3 steps
1: 125 °C
2: potassium hydroxide / butan-1-ol / 120 °C / Inert atmosphere
3: hydrogenchloride / acetone; water / 0 - 10 °C
With hydrogenchloride; potassium hydroxide; In water; acetone; butan-1-ol;
|
|
|
Multi-step reaction with 2 steps
1.1: 14 h / 157 °C
1.2: 5 - 25 °C
2.1: hydrogenchloride / water / 8 h / 2 - 102 °C
With hydrogenchloride; In water;
|
|
|
Multi-step reaction with 2 steps
1: formamide / 8 h / 155 °C
2: hydrogenchloride / water / 8 h / 2 - 102 °C
With hydrogenchloride; formamide; In water;
|
|
|
Multi-step reaction with 4 steps
1.1: 14 h / 157 °C
1.2: 5 - 25 °C
2.1: hydrogenchloride / water / 21 h / 28 °C
3.1: 10 h / 152 °C
4.1: hydrogenchloride / water / 8 h / 2 - 102 °C
With hydrogenchloride; In water;
|
|
|
Multi-step reaction with 4 steps
1: formamide / 8 h / 155 °C
2: hydrogenchloride / water / 21 h / 28 °C
3: 10 h / 152 °C
4: hydrogenchloride / water / 8 h / 2 - 102 °C
With hydrogenchloride; formamide; In water;
|
|
|
Multi-step reaction with 2 steps
1.1: phosphoric acid / 3.75 - 4.33 h / 20 - 87 °C
1.2: 35 - 45 °C / pH 5.5 - 7
2.1: potassium hydroxide; butan-1-ol / 10.33 - 10.5 h / 20 - 132 °C
2.2: 0.67 h / 20 - 25 °C
With potassium hydroxide; phosphoric acid; butan-1-ol;
|
|
|
Multi-step reaction with 2 steps
1.1: sulfuric acid / 15.5 - 16 h / 10 - 35 °C
1.2: 20 °C
2.1: sodium hydroxide / diethylene glycol / 10 h / Heating / reflux
2.2: 2 h / 10 - 15 °C
With sodium hydroxide; sulfuric acid; In diethylene glycol;
|
|
|
Multi-step reaction with 3 steps
1.1: sulfuric acid / 22 h / 10 - 25 °C
1.2: 1.5 h / 25 - 35 °C
2.1: ammonia / dichloromethane; water / 0.5 h / 25 - 30 °C / pH 9.5 - 10.0
3.1: sodium hydroxide / diethylene glycol / 10 h / Heating / reflux
3.2: 2 h / 10 - 15 °C
With sodium hydroxide; sulfuric acid; ammonia; In dichloromethane; water; diethylene glycol;
|
|
|
Multi-step reaction with 3 steps
1: 6 h / 70 - 160 °C / Inert atmosphere
2: potassium hydroxide / butan-1-ol / 10.5 h / 130 °C / Inert atmosphere
3: hydrogenchloride / water; acetone / 5 h / -5 - 30 °C
With hydrogenchloride; potassium hydroxide; In water; acetone; butan-1-ol;
|
|
|
Multi-step reaction with 2 steps
1.1: 8 h / 130 °C
2.1: potassium hydroxide; butan-1-ol / 130 °C
2.2: pH 8 - 9
With potassium hydroxide; butan-1-ol;
|
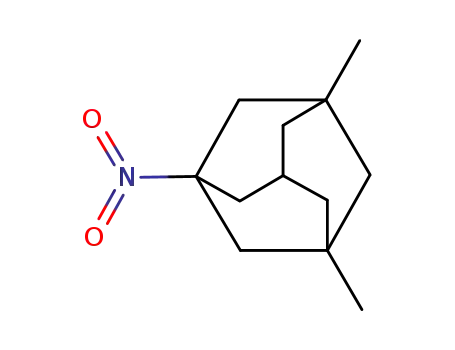
1-nitro-3,5-dimethyl-adamantane

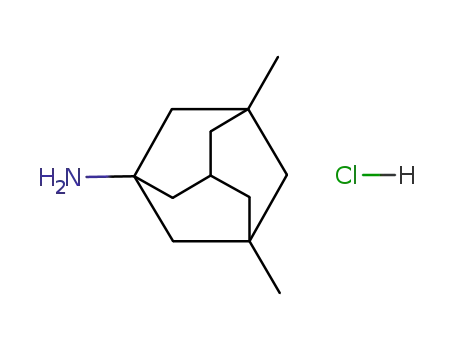
memantine hydrochloride
| Conditions | Yield |
|---|---|
|
1-nitro-3,5-dimethyl-adamantane; With hydrogen; In ethanol; at 80 ℃; for 0.5h; under 750.075 - 18751.9 Torr; Autoclave;
With hydrogenchloride; for 0.5h;
|
97.4% |
|
1-nitro-3,5-dimethyl-adamantane; With iron; acetic acid; at 20 ℃; for 4.5h;
With hydrogenchloride; In ethyl acetate; for 0.5h;
|
449.2 g |
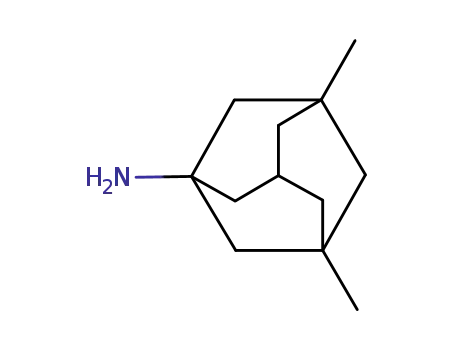
memantine*
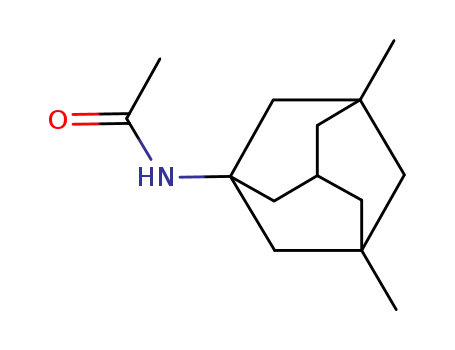
1-acetamido-3,5-dimethyladamantane
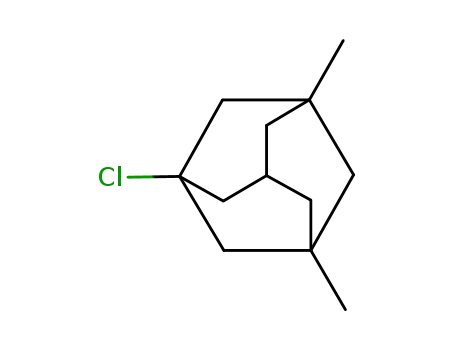
1-chloro-3,5-dimethyl-adamantane
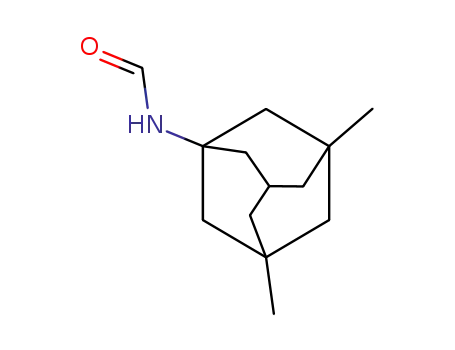
1-formamido-3,5-dimethyltricyclo[3.3.1.13,7]decane

memantine*
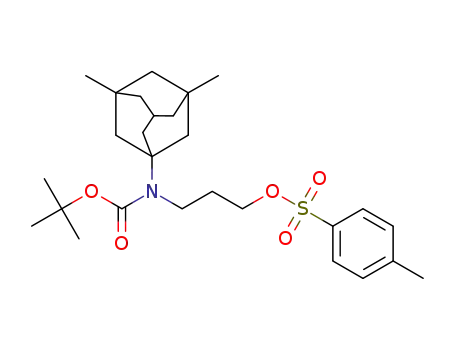
3-((tert-butoxycarbonyl)(3,5-dimethyladamantan-1-yl)amino)propyl 4-methylbenzenesulfonate
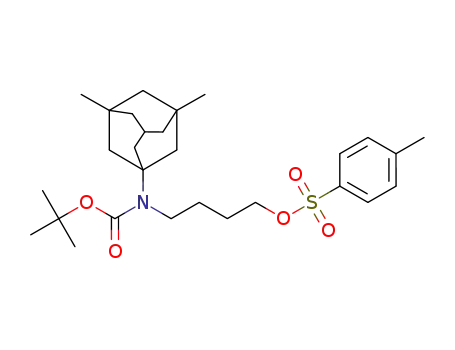
4-((tert-butoxycarbonyl)(3,5-dimethyladamantan-1-yl)amino)butyl 4-methylbenzenesulfonate
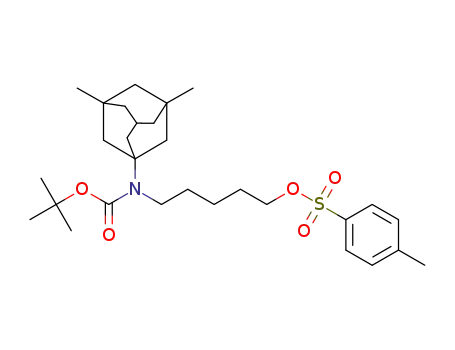
5-((tert-butoxycarbonyl)(3,5-dimethyladamantan-1-yl)amino)pentyl 4-methylbenzenesulfonate