Your Location:Home > Products > Pharmaceutical > Icaridin
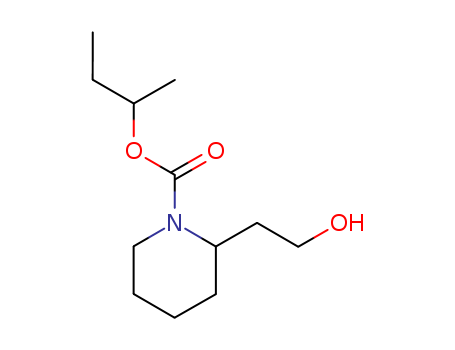

CasNo: 119515-38-7
Molecular Formula: C12H23NO3
Appearance: Colourless liquid
|
119515-38-7 Name |
|
|
Name |
lcaridin |
|
Synonym |
sec-Butyl 2-(2-hydroxyethyl)piperidine-1-carboxylate; 1-(1-methylpropoxycarbonyl)-2-(2-hydroxyethyl)piperidine;1-methylpropyl2-(2-hydroxyethyl)-1-piperidinecarboxylate;2-(2-hydroxyethyl)-1-piperidinecarboxylicaci1-methylpropylester;HYDROXYETHYL ISOBUTYL PIPERIDINE CARBOXYLATE;BAYREPELVE;2-(2-Hydroxyethyl)-1-piperidinecarboxylic Acid 1-Methyl-propyl Ester;Bayrepe;Picaridin |
|
119515-38-7 Biological Activity |
|
|
Related Catalog |
Research Areas >> Infection Signaling Pathways >> Others >> Others |
|
119515-38-7 Chemical & Physical Properties |
|
|
Melting point |
below -170ºC |
|
Boiling point |
330.9±15.0 °C at 760 mmHg |
|
Density |
1.0±0.1 g/cm3 |
|
Molecular Formula |
C12H23NO3 |
|
Molecular Weight |
229.316 |
|
Flash Point |
153.9±20.4 °C |
|
PSA |
49.77000 |
|
LogP |
1.56 |
|
Exact Mass |
229.167801 |
|
Vapour Pressure |
0.0±1.6 mmHg at 25°C |
|
Index of Refraction |
1.478 |
Icaridin, also known as picaridin or KBR 3023, is a derivative of a natural compound from pepper plants that possess high repellent effectiveness, being the most recommended active compound by the WHO. Picaridin is an insect repellent which inhibits A. aegypti odorant receptor 2 (AaOR2) or AaOR8 in the presence of their odorant activators, indole and octenol, respectively, expressed in Xenopus oocytes (IC50s = 1,452 and 1,911 μM, respectively). It acts on certain olfactory receptor cell types to reduce the activating or attracting effect of odor sources. Insect repellent
InChI:InChI=1/C12H23NO3/c1-3-10(2)16-12(15)13-8-5-4-6-11(13)7-9-14/h10-11,14H,3-9H2,1-2H3
The materials used in this work were Geraniol (GER), Icaridin (ICA), Zein, Pluronic F-68, MTT (3-(4,5-dimethylthiazolyl-2)− 2,5-diphenyltetrazolium bromide), Cell Counting Kit-8 (Sigma-Aldrich). The studies of the expression of genes related to the signaling of inflammatory processes showed that TPH-1 cells in direct contact with the compounds (2D culture) present different expressions compared to those exposed in the co-culture.
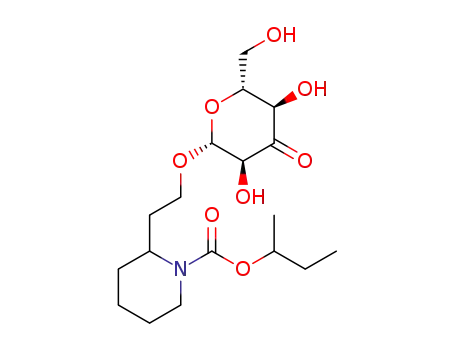
sec-butyl 2-(2-(((2R,3S,5R,6R)-3,5-dihydroxy-6-(hydroxymethyl)-4-oxotetrahydro-2H-pyran-2-yl)oxy)ethyl)piperidine-1-carboxylate

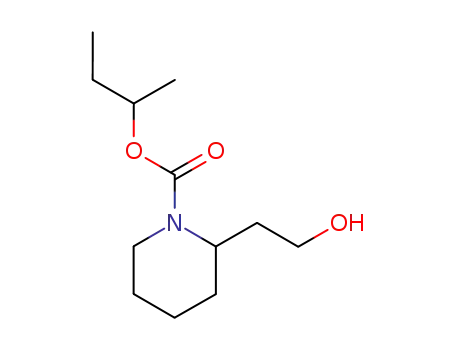
icaridin
| Conditions | Yield |
|---|---|
|
With water-d2; In aq. buffer; at 32 ℃; for 50h; pH=4; pH-value;
|
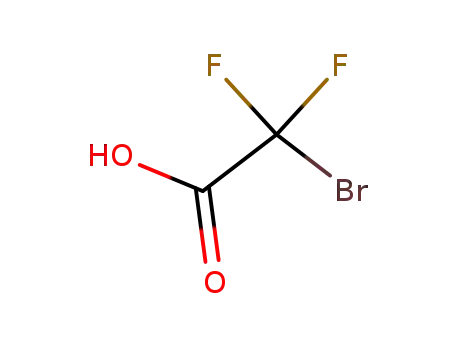
bromodifluoroacetic acid


icaridin

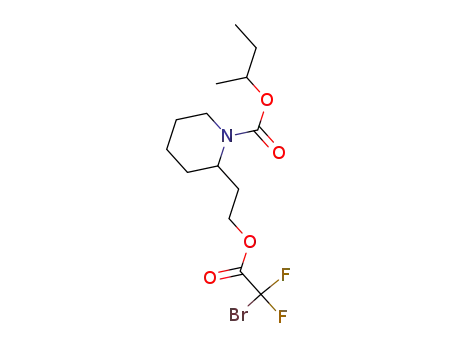
C14H22BrF2NO4
| Conditions | Yield |
|---|---|
|
With oxalyl dichloride; triethylamine; N,N-dimethyl-formamide; at 0 - 20 ℃; Inert atmosphere;
|
66% |