Your Location:Home > Products > Fine Chemicals > 4'-Ethylpropiophenone
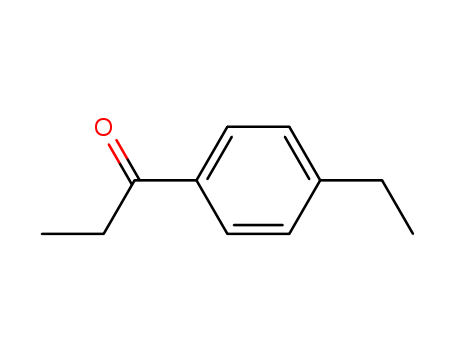

CasNo: 27465-51-6
Molecular Formula: C11H14O
|
27465-51-6 Name |
|
|
Name |
4'-Ethylpropiophenone |
|
Synonym |
1-(4-Ethylphenyl)propanone;1-Propanone,1-(4-ethylphenyl)-;Theethylbenzeneacetone;P-ETHYLPROPIOPHENONE;1-(4-ETHYL-PHENYL)-PROPAN-1-ONE;4'-ETHYLPROPIOPHENONE;4-ETHYLPROPIOPHENONE;4'-ETHYLPROPIOPHENONE 96+% |
|
27465-51-6 Chemical & Physical Properties |
|
|
Boiling point |
257.5±19.0 °C at 760 mmHg |
|
Density |
1.0±0.1 g/cm3 |
|
Molecular Formula |
C11H14O |
|
Molecular Weight |
162.228 |
|
Flash Point |
103.2±16.5 °C |
|
PSA |
17.07000 |
|
LogP |
3.19 |
|
Exact Mass |
162.104462 |
|
Vapour Pressure |
0.0±0.5 mmHg at 25°C |
|
Index of Refraction |
1.504 |
4-ethylpropiophenone (EPP), Clear colorless liquid, is An impurity of Eperisone (E565800) as muscle relaxant and analgesic. It is a synthetic chemical that belongs to the class of piperidine hydrochloride.
InChI:InChI=1/C11H14O/c1-3-10-4-6-11(7-5-10)8-9(2)12/h4-7H,3,8H2,1-2H3
The produced carbonyl compounds can be further transformed into α-ketoamides, homoallylic alcohols and oximes in a one-pot reaction.
The trialkylboranes generated in situ by hydroboration of olefins with BH3 or 9-BBN performed similarly to those separately prepared, making this protocol more practical.
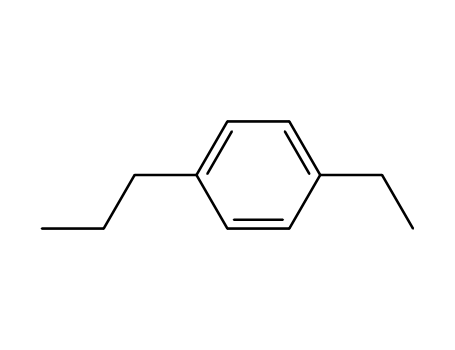
p-(n-propyl)ethylbenzene

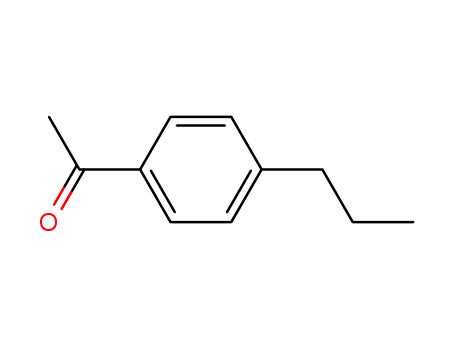
1-(4-propylphenyl)ethan-1-one

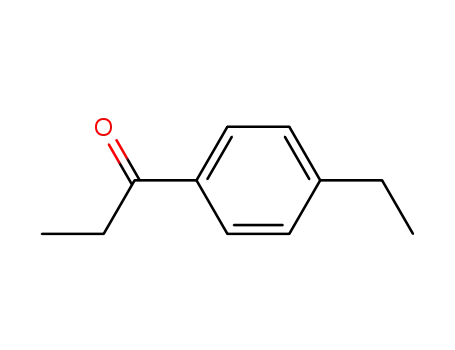
1-(4-ethylphenyl)-1-propanone
| Conditions | Yield |
|---|---|
|
With pyridine; N-hydroxyphthalimide; tetrabutylammonium tetrafluoroborate; oxygen; In 2,2,2-trifluoroethanol; acetonitrile; at 35 ℃; Overall yield = 65 percent; Electrolysis;
|
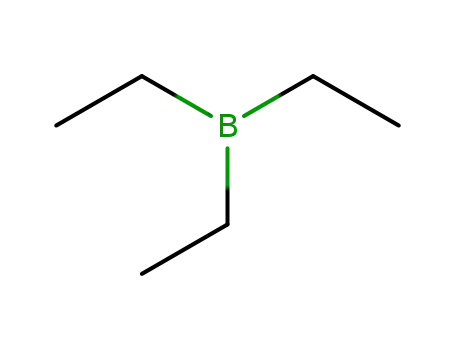
triethyl borane

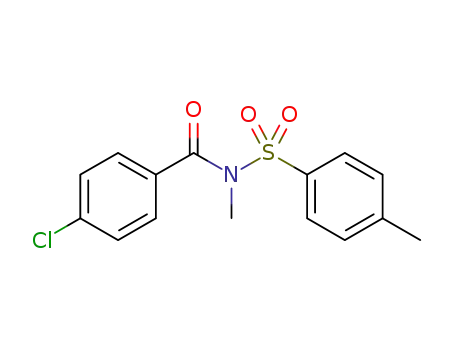
N-methyl-N-tosyl-p-chlorobenzamide


1-(4-ethylphenyl)-1-propanone
| Conditions | Yield |
|---|---|
|
With [1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene](3-chloropyridyl)palladium(ll) dichloride; potassium carbonate; In tetrahydrofuran; tert-butyl methyl ether; at 20 ℃; for 24h; Inert atmosphere; Schlenk technique;
|
88% |
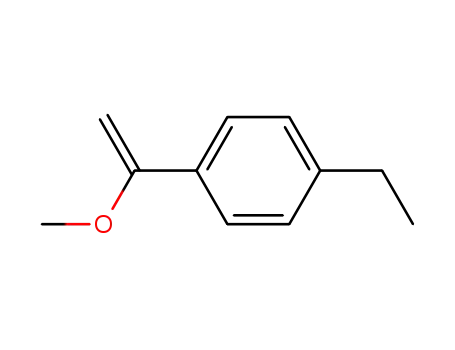
[1-(4-ethyl-phenyl)-vinyl]-methyl ether
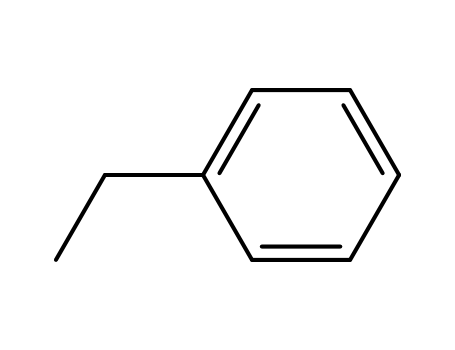
ethylbenzene
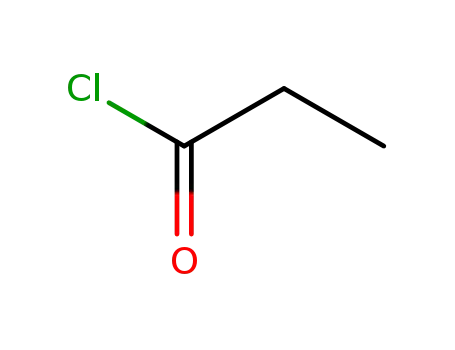
propionyl chloride
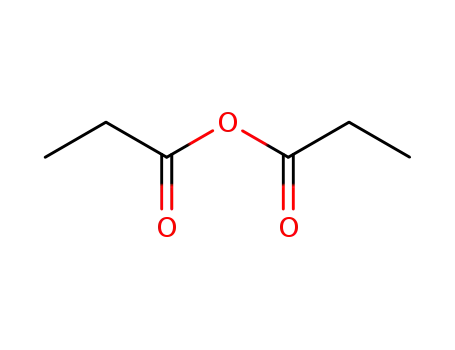
propionic acid anhydride
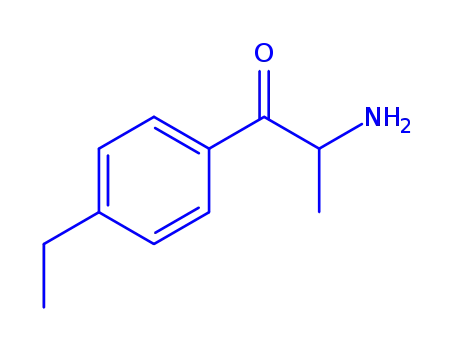
α-Amino-4-ethyl-propiophenon

p-(n-propyl)ethylbenzene
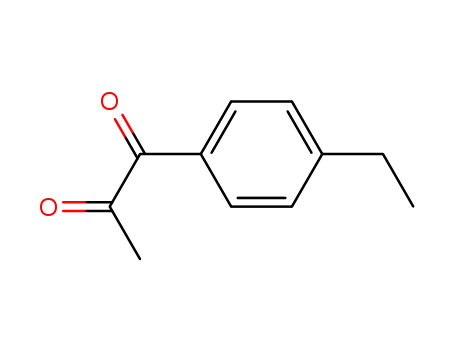
1-(4-ethylphenyl)propane-1,2-dione
1-(4-ethyl-phenyl)-propan-1-one oxime