Your Location:Home > Products > Benzeneacetic acid, a-methyl-4-[(2-oxocyclopentyl)methyl]-
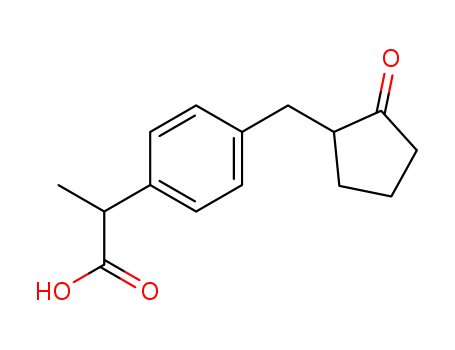

CasNo: 68767-14-6
Molecular Formula: C15H18O3
Appearance: Colorless oily matter
|
68767-14-6 Name |
|
|
Name |
Loxoprofen |
|
Synonym |
IOXOPROFEN;2-[4-[(2-Oxocyclopentan-1-yl)methyl]phenyl]propionic acid;KOLOXO;LOXOPROFEN;2-[4-(2-OXO-CYCLOPENTYLMETHYL)-PHENYL]-PROPIONIC ACID;A-METHYL-4-[(2-OXOCYCLOPENTYL)METHYL]BENZENEACETIC ACID;α-methyl-4-[(2-oxocyclopentyl)methyl]benzeneacetic acid;2-{4-[(2-oxocyclopentyl)methyl]phenyl}propanoic acid |
|
68767-14-6 Biological Activity |
|
|
Description |
Loxoprofen is a non-steroidal anti-inflammatory drug.Target: COXLoxoprofen is a non-steroidal anti-inflammatory drug in the propionic acid derivatives group, which also includes ibuprofen and naproxen among others. Loxoprofen is a non-selective cyclooxygenase inhibitor, and works by reducing the synthesis of prostaglandins from arachidonic acid. |
|
Related Catalog |
Research Areas >> Inflammation/Immunology |
|
Target |
COX-1:6.5 μM (IC50, in human whole blood) COX-2:13.5 μM (IC50, in human whole blood) |
|
References |
[1]. Riendeau D, et al. Evaluation of loxoprofen and its alcohol metabolites for potency and selectivity of inhibition of cyclooxygenase-2. Bioorg Med Chem Lett. 2004 Mar 8;14(5):1201-3. |
|
68767-14-6 Chemical & Physical Properties |
|
|
Melting point |
108.5 - 111ºC |
|
Boiling point |
417.9±20.0 °C at 760 mmHg |
|
Density |
1.2±0.1 g/cm3 |
|
Molecular Formula |
C15H18O3 |
|
Molecular Weight |
246.302 |
|
Flash Point |
220.7±18.3 °C |
|
PSA |
54.37000 |
|
LogP |
1.87 |
|
Exact Mass |
246.125595 |
|
Vapour Pressure |
0.0±1.0 mmHg at 25°C |
|
Index of Refraction |
1.568 |
Loxoprofen is a non-selective nonsteroidal anti-inflammatory drug (NSAID) that has been effective in reducing atherosclerosis in mice by reducing inflammation. Loxoprofen becomes active after metabolism in the body and inhibits the activation of cyclooxygenase.
Definition
ChEBI: A monocarboxylic acid that is propionic acid in which one of the hydrogens at position 2 is substituted by a 4-[(2-oxocyclopentyl)methyl]phenyl group. A prodrug that is rapidly converted to its active trans-alcohol metabolite following ora administration.
InChI:InChI=1/C15H18O3/c1-10(15(17)18)12-7-5-11(6-8-12)9-13-3-2-4-14(13)16/h5-8,10,13H,2-4,9H2,1H3,(H,17,18)
The invention discloses a novel preparation method of loxoprofen (I). The reaction process comprises the following steps: a compound (VII) and a compound (VIII) are subjected to a condensation reaction to obtain an intermediate (IX), the intermediate (IX) is subjected to a hydrogenation reduction reaction in a mixed solvent of acid and alcohol to obtain an intermediate (X), and the intermediate (X) is subjected to a hydrolysis reaction to obtain loxoprofen (I).
The invention provides a preparation method of an aryl propionic acid compound, substituted or unsubstituted C3-C12 naphthenic base, substituted carbonyl containing C6-C24 aryl or substitutedaryl, substituted carbonyl containing C3-C12 heterocyclic radical or substituted heterocyclic radical, phenyl, substituted phenyl, naphthyl and substituted naphthyl.
The synthetic method has the advantages that the synthetic route is short, simple in process and convenient to operate, no corrosive strong acid is used in the synthesis process, and the reaction control temperature in the synthesis process is relatively mild; and the synthetic method has the characteristics of no product carbonization, no corrosion to equipment, safe and environment-friendly synthesis process, suitability for industrial production and the like, and the finally obtained product loxoprofen has high yield which can reach 65.8-73.3% and purity of 99.54-99.69% from the beginning of the compound VIII.
The invention belongs to the field of synthesis of organic matters and specifically relates to a new method for synthesizing loxoprofen sodium. The synthesis method is characterized by taking 2-(4-bromomethyl) phenylpropionic acid as a raw material and performing a 4-step reaction to prepare the loxoprofen sodium. The new method for synthesizing the loxoprofen sodium adopted by the invention has the effects that the yield is increased and the industrial prospect is good.
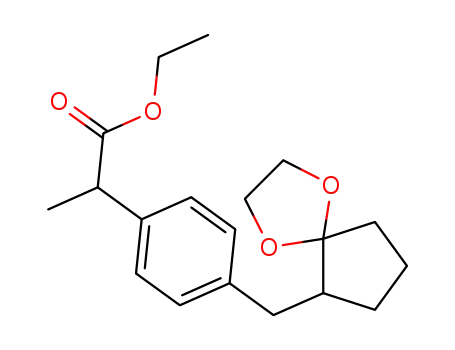
C19H26O4

loxoprofen
| Conditions | Yield |
|---|---|
|
C19H26O4; With hydrogenchloride; In methanol; water; at 0 ℃; Reflux;
In tert-butyl methyl ether; at 0 ℃; Reflux;
|
85% |
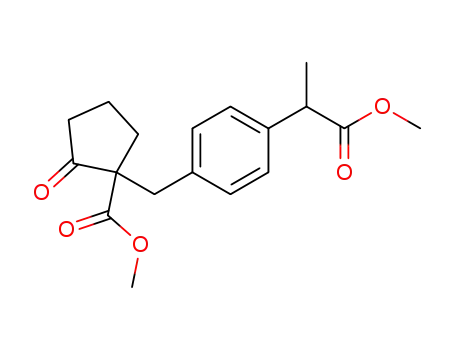
C18H22O5


loxoprofen
| Conditions | Yield |
|---|---|
|
With sulfuric acid; acetic acid; In water; at 110 ℃; for 2h;
|
94.3% |
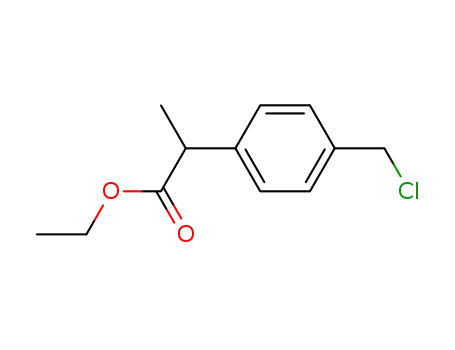
2-(4-chloromethylphenyl)-propionic ethyl ester
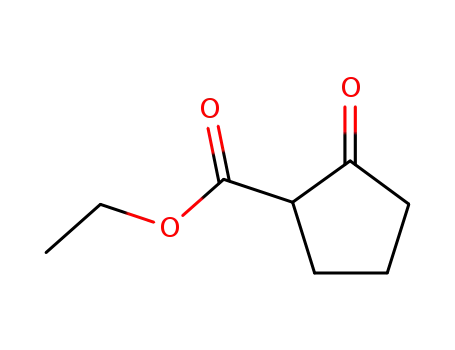
2-ethoxycarbonyl-1-cyclopentanone
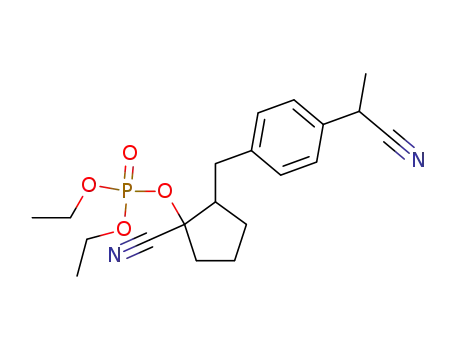
Phosphoric acid 1-cyano-2-[4-(cyano-methyl-methyl)-benzyl]-cyclopentyl ester diethyl ester
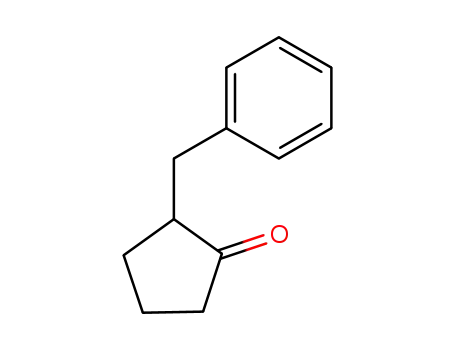
2-benzylcyclopentanone
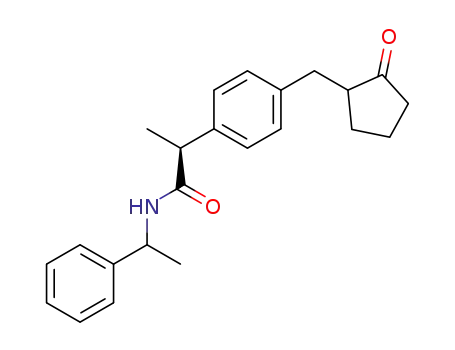
(-)-N-<(1S)-1-phenylethyl>-(2S)-2-<4-(2-oxocyclopentylmethyl)phenyl>propionamide
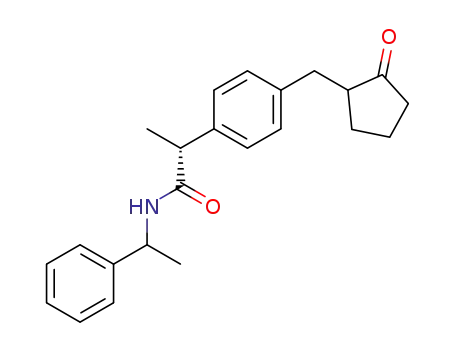
(-)-N-<(1S)-1-phenylethyl>-(2R)-2-<4-(2-oxocyclopentylmethyl)phenyl>propionamide
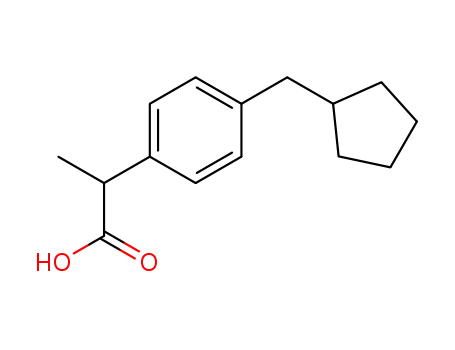
2-<4-(cyclopentylmethyl)phenyl>propionic acid
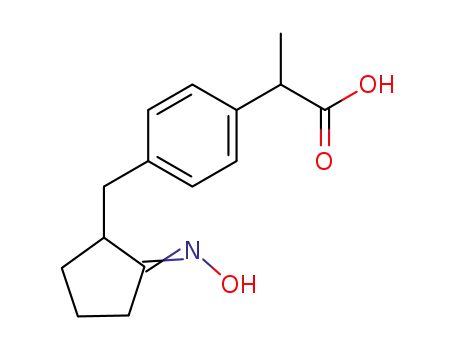
2-(4-{2-[(E)-Hydroxyimino]-cyclopentylmethyl}-phenyl)-propionic acid