Your Location:Home > Products > Cannabis > Olivetol Dimethyl Ether
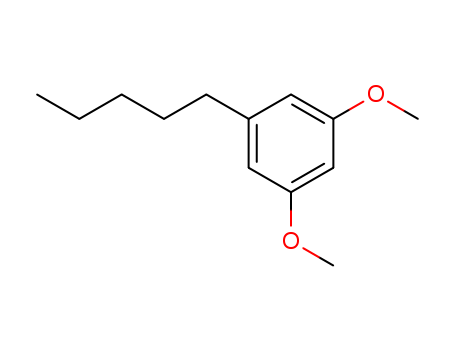

CasNo: 22976-40-5
Molecular Formula: C13H20O2
|
22976-40-5 Name |
|
|
Name |
Olivetol Dimethyl Ether |
|
Synonym |
Olivetol Dimethyl Ether;1,3-DiMethoxy-5-pentylbenzene;1,3-DiMethoxy-5-pentyl-benzene;3,5-DiMethoxyaMylbenzene;5-Pentylresorcinol DiMethyl Ether;Di-O-Methylolivetol;Benzene, 1,3-dimethoxy-5-pentyl-;dimethoxyolivetol |
|
22976-40-5 Chemical & Physical Properties |
|
|
Boiling point |
296.8±20.0 °C at 760 mmHg |
|
Density |
0.9±0.1 g/cm3 |
|
Molecular Formula |
C13H20O2 |
|
Molecular Weight |
208.297 |
|
Flash Point |
107.8±21.3 °C |
|
PSA |
18.46000 |
|
LogP |
4.51 |
|
Exact Mass |
208.146332 |
|
Vapour Pressure |
0.0±0.6 mmHg at 25°C |
|
Index of Refraction |
1.486 |
Olivetol Dimethyl Ether, Off-White Solid, An intermediate in various syntheses of tetrahydrocannabinol. The synthesis of olivetol dimethyl ether is representative.
InChI=1S/C13H20O2/c1-4-5-6-7-11-8-12(14-2)10-13(9-11)15-3/h8-10H,4-7H2,1-3H3
InChIKey: LSPSEUBXQFHRGA-UHFFFAOYSA-N
The regioselectivity of deprotonation/alkylation reactions of η6-1,3-dimethoxybenzene-Cr(CO)3 (5), η6-1,3-dimethoxy-5-methylbenzene-Cr(CO)3 (6) and 2-substituted derivatives of these compounds was investigated.
We became interested in the synthesis of 5-alkylresorcinols such as olivetol and olivetolic ester derivatives for several reasons. In this case, the mono methyl ether 4 and methyl olivetolate-dimethyl ether 5 were obtained in high yield and no trace of methyl olivetolate 3 was observed.
It commenced with the preparation of homocuprate olivetol 20 from the corresponding lithiated olivetol dimethyl ether. Cuprate 20 was coupled in an SN2′-fashion to p-mentha-2,8-dien-1-ol acetate (19) using boron trifluoride etherate and afforded (−)-trans-CBD dimethyl ether 21 in 78% yield.
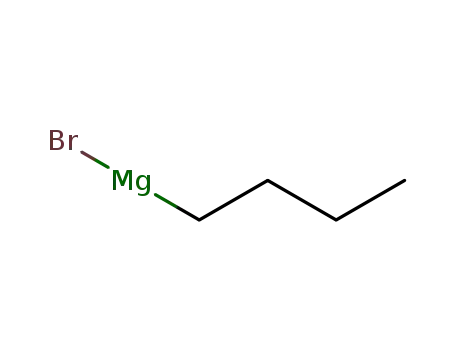
n-butyl magnesium bromide

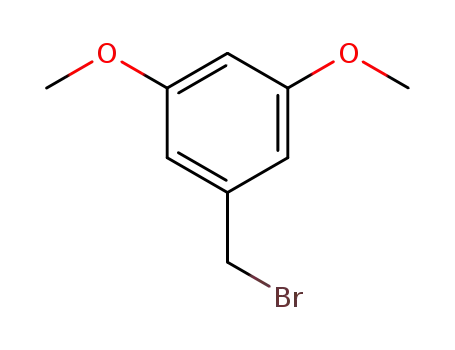
3,5-dimethoxybenzyl bromide

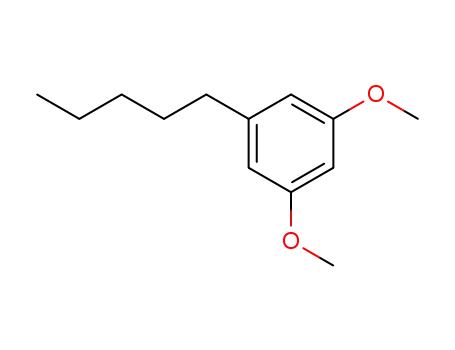
1,3-dimethoxy-5-pentylbenzene

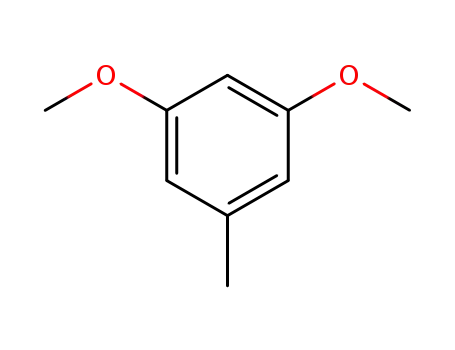
1,3-dimethoxy-5-methylbenzene
| Conditions | Yield |
|---|---|
|
With dilithium tetrachlorocuprate;
|
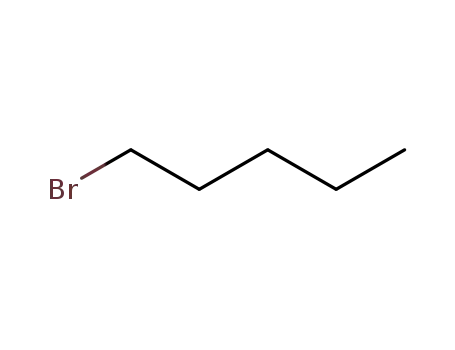
1-Bromopentane

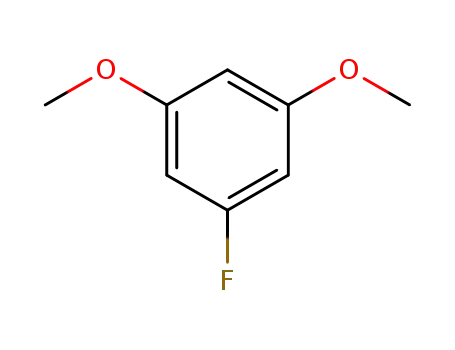
3,5-dimethoxyfluorobenzene


1,3-dimethoxy-5-pentylbenzene
| Conditions | Yield |
|---|---|
|
With lithium; for 0.5h;
|
60% |
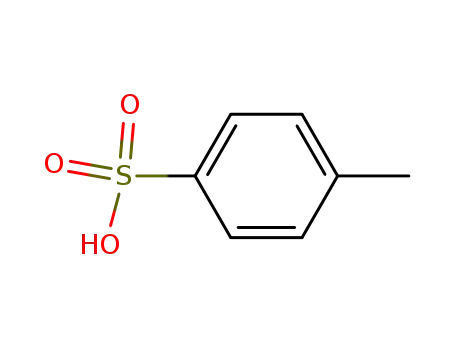
toluene-4-sulfonic acid
benzene
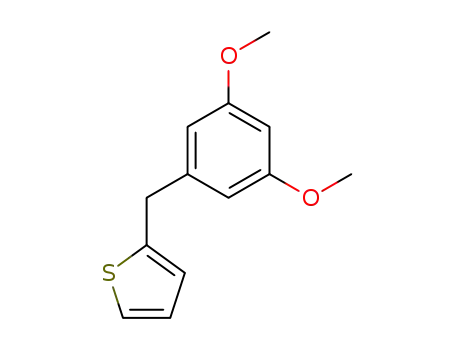
2-(3,5-dimethoxy-benzyl)-thiophene
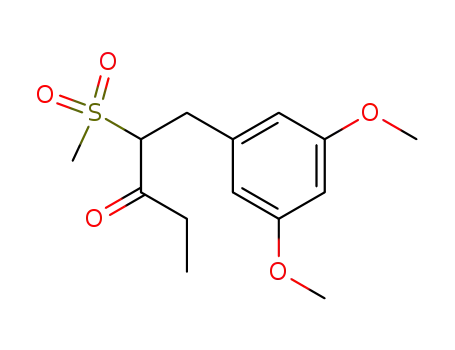
1-(3,5-Dimethoxyphenyl)-2-methylsulfonyl-pentan-3-on
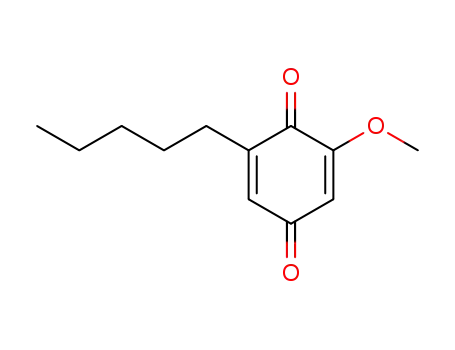
primine
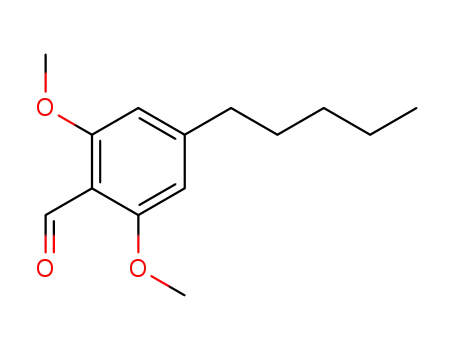
2,6-dimethoxy-4-pentylbenzaldehyde
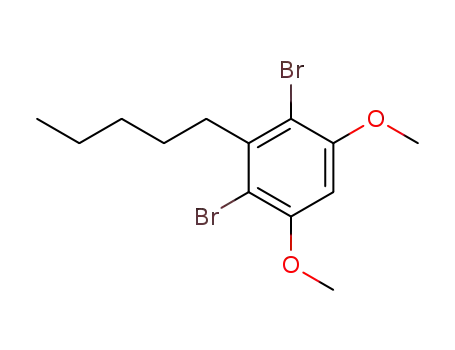
2,4-dibromo-1,5-dimethoxy-3-pentylbenzene
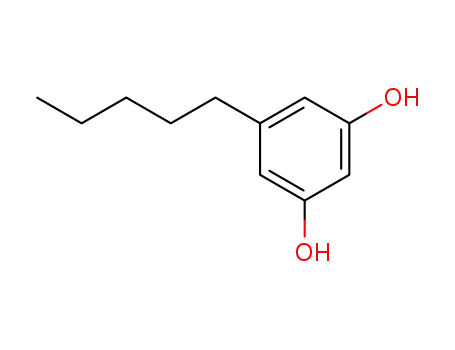
Olivetol