Your Location:Home > Products > Cannabis > 3,5-dihydroxy-1-isopropylbenzene
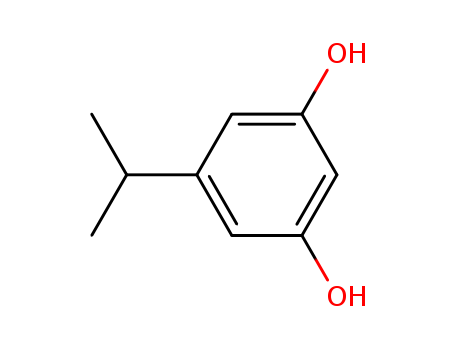

CasNo: 34993-66-3
Molecular Formula: C9H12O2
|
34993-66-3 Name |
|
|
Name |
3,5-dihydroxy-1-isopropylbenzene |
|
Synonym |
3,5-dihydroxy-1-isopropylbenzene,5-Isopropyl-resorcin,5-isopropyl-resorcinol,5-isopropylbenzene-1,3-diol,m-Isopropylresorcin |
The chemical formula of 1,3-Benzenediol, 5-(1-methylethyl)- is C9H12O2 which containing 9 Carbon atoms,12 Hydrogen atoms and 2 Oxygen atoms,and the molecular weight of 1,3-Benzenediol, 5-(1-methylethyl)- is 152.193.
|
34993-66-3 Chemical & Physical Properties |
|
|
Molecular Formula |
C9H12O2 |
|
Molecular Weight |
152.19000 |
|
PSA |
40.46000 |
|
LogP |
2.22120 |
|
Exact Mass |
152.08400 |
The conversion of substituted 1,3-cyclohexanediones to the alkyl ethers of resorcinol using a Pd/C-ethylene system is reported. In these reactions, ethylene works as a hydrogen acceptor. The efficient synthesis of resveratrol was achieved using this protocol as a key step. In addition, the direct formation of substituted resorcinols was carried out by adding K2CO3 into the reaction media.
Based on our former development candidate BAY 38-1315, optimization efforts led to the discovery of a novel chemical class of orally active cholesteryl ester transfer protein (CETP) inhibitors. The chromanol derivative 19b is a highly potent CETP inhibitor with favorable pharmacokinetic properties suitable for clinical studies. Chemical process optimization furnished a robust synthesis for a kilogram-scale process.
The first systematic study on the aerobic oxidation of 1,3,5- triisopropylbenzene was examined by the use of N-hydroxyphthalimide (NHPI) as a key catalyst. This oxidation provides a facile method for preparing phenol derivatives bearing an isopropyl moiety, which can be used as pharmaceutical starting materials.
Michael-type additions of phenylsulphinylacetate esters to αβ-unsaturated ketones produce cyclohexane-1,3-dione derivatives, which, after thermal elimination of benzenesulphenic acid, give the corresponding 5-substituted resorcinols, such as olivetol.The scope of this entry into other aromatic systems, such as 3,5-disubstituted and 2,3,5-trisubstituted phenols and orsellinic acid has been explored.
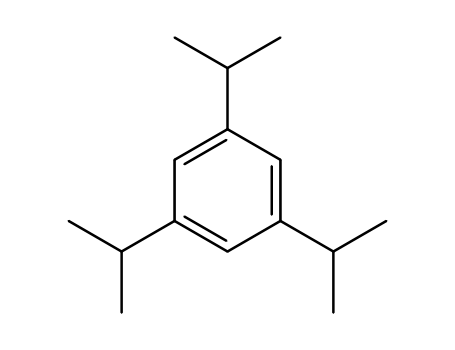
1,3,5-triisopropyl benzene

3,5-dihydroxyphenol

3,5-diisopropylphenol

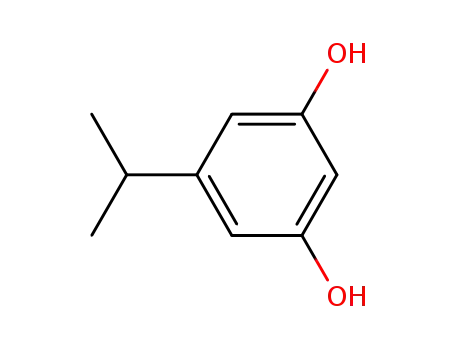
3,5-dihydroxy-1-isopropylbenzene
| Conditions | Yield |
|---|---|
|
1,3,5-triisopropyl benzene; With N-hydroxyphthalimide; 2,2'-azobis(isobutyronitrile); oxygen; In acetonitrile; at 75 ℃; for 2h; under 760 Torr;
With sulfuric acid; In acetonitrile; at 20 ℃; for 2h; Title compound not separated from byproducts;
|
|
|
1,3,5-triisopropyl benzene; With N-hydroxyphthalimide; 2,2'-azobis(isobutyronitrile); oxygen; In acetonitrile; at 75 ℃; for 24h; under 22800 Torr;
With sulfuric acid; In acetonitrile; at 20 ℃; for 2h; Title compound not separated from byproducts;
|


3,5-dihydroxy-1-isopropylbenzene

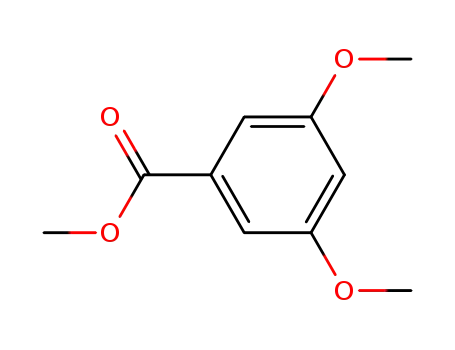
methyl 3,5-dimethoxybenzoate
| Conditions | Yield |
|---|---|
|
|
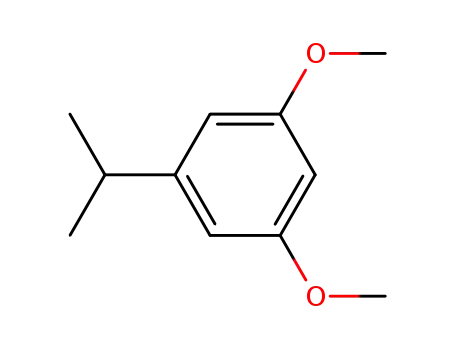
5-isopropyl-1,3-dimethoxybenzene
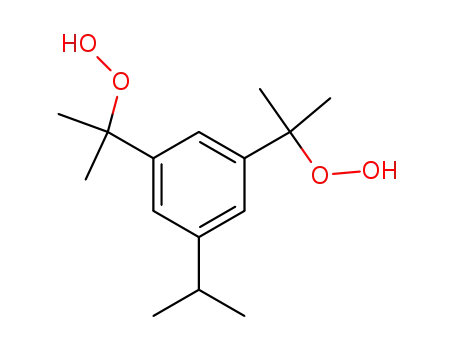
1,3-bis-(α-hydroperoxy-isopropyl)-5-isopropyl-benzene
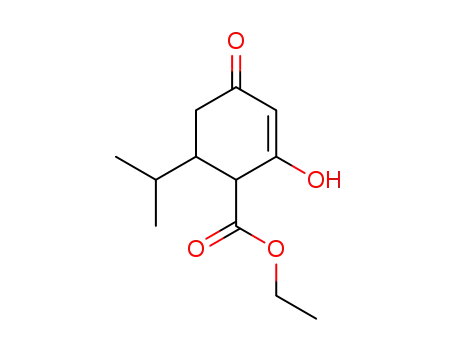
2-Hydroxy-6-isopropyl-4-oxo-cyclohex-2-enecarboxylic acid ethyl ester
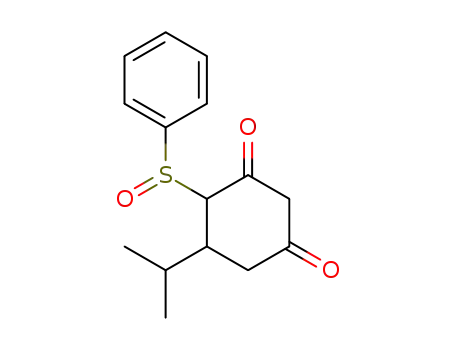
4-Benzenesulfinyl-5-isopropyl-cyclohexane-1,3-dione
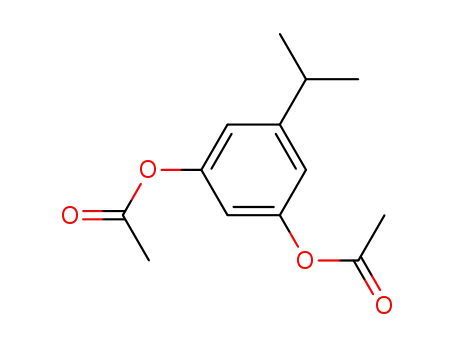
3-isopropyl-1,5-diacetoxybenzene
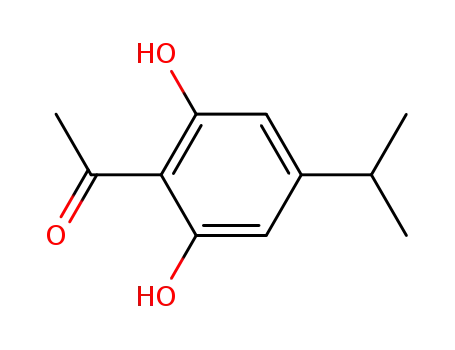
2',6'-dihydroxy-4'-iso-propylacetophenone
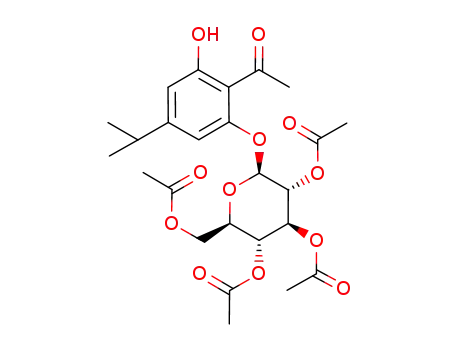
2',6'-dihydroxy-4'-iso-propylacetophenone 2'-O-(2,3,4,6-O-tetraacetyl)-β-D-glucopyranoside
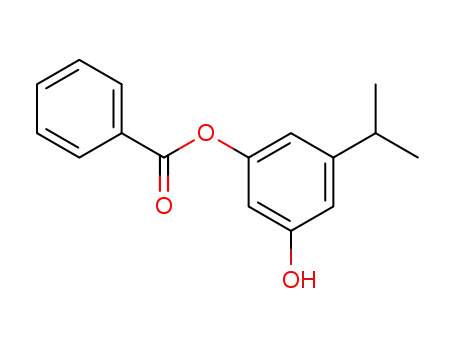
3-hydroxy-5-benzoyloxy-1-isopropylbenzene