Your Location:Home > Products > Cannabis > Ethyl Olivetolate
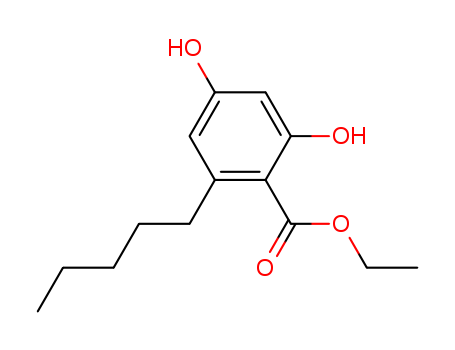

CasNo: 38862-65-6
Molecular Formula: C14H20O4
|
38862-65-6 Name |
|
|
Name |
Ethyl Olivetolate |
|
Synonym |
Ethyl Olivetolate;BENZOIC ACID, 2,4-DIHYDROXY-6-PENTYL-, ETHYL ESTER;ethyl 2,4-dihydroxy-6-pentylbenzoate;2,4-dihydroxy-6-n-pentylbenzoic acid ethyl ester;ethyl 2,4-dihydroxy-6-pentylbenzoate(Ethyl olivetolate);2,4-Dihydroxy-6-pentyl-benzoesaeure-aethylester |
|
Chemical & Physical Properties |
|
|
Melting point |
69 °C |
|
Boiling point |
420.4±30.0 °C(Predicted) |
|
Density |
1.131±0.06 g/cm3(Predicted) |
|
Molecular Formula |
C14H20O4 |
|
Molecular Weight |
252.30600 |
|
PSA |
66.76000 |
|
LogP |
3.00720 |
|
Exact Mass |
252.13600 |
Ethyl Olivetolate is used for preparation of crystalline Cannabidiol for pharmaceutical formulations. Olivetol and ethyl olivetolate which are cannabinoid precursors were not biotransformed to cannabinoids.
A new enzyme, geranylpyrophosphate:olivetolate geranyltransferase (GOT), the first enzyme in the biosynthesis of cannabinoids could be detected in extracts of young leaves of Cannabis sativa. Influence of substrates, cofactors and inhibitors on the activity of geranylpyrophosphate:olivetolate geranyltransferase.
The present invention relates to a process for preparation of a delta-9-tetrahydrocannabinol compound or derivative thereof involving treating a first intermediate compound with an organoaluminum-based Lewis acid catalyst, under conditions effective to produce the delta-9-tetrahydrocannabinol compound or derivative thereof. The present invention also relates to a compound of the formula: where R8, R9, and R10 are the same or different and independently selected from the group consisting of H, substituted or unsubstituted alkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, or halo, with R1, R2, and R3 defined herein.
In order to clarify the structure-activity relationship of 4-O-methylcryptochlorophaeic acid (1), which is a lichen meta-depside and a potent inhibitor of prostaglandin (PG) biosynthesis found in our previous screening work, arylcarboxylic acids (5-8) corresponding to the monomeric moieties of 4-O-methylcryptochlorophaeic acid (1) were synthesized and tested for inhibitory effect against PG biosynthesis by an enzyme system prepared from rabbit renal medulla. Based on these findings, a new active site model of fatty acid cylooxygenase is proposed in order to explain the inhibition by the meta-depside and acidic non-steroidal antiinflammatory drugs.
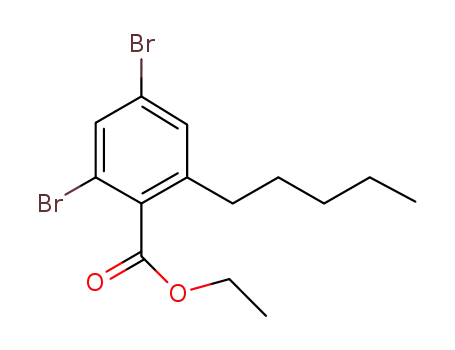
C14H18Br2O2

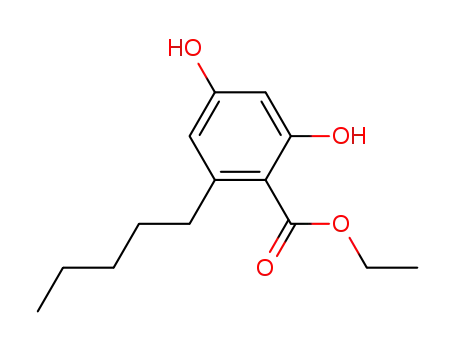
2,4-dihydroxy-6-n-pentylbenzoic acid ethyl ester
| Conditions | Yield |
|---|---|
|
With water; sodium citrate; 5% palladium over charcoal; In ethanol; at 60 ℃; for 6h;
|
72% |
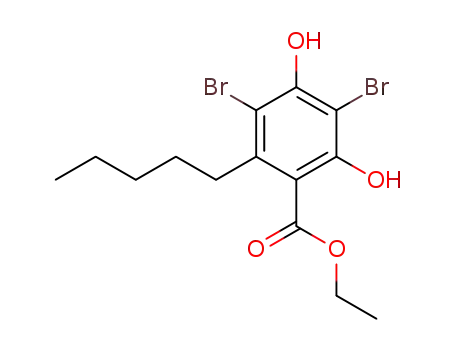
3,5-dibromo-2,4-dihydroxy-6-n-pentylbenzoic acid ethyl ester


2,4-dihydroxy-6-n-pentylbenzoic acid ethyl ester
| Conditions | Yield |
|---|---|
|
With hydrogen; sodium acetate; palladium on activated charcoal; In acetic acid; Ambient temperature;
|
92% |
|
With sodium hydroxide; palladium; Hydrogenation;
|
|
|
With hydrogen; palladium on activated charcoal;
|
|
|
With palladium 10% on activated carbon; hydrogen; sodium citrate; In ethanol; water; at 55 - 65 ℃; for 14h; under 3800.26 Torr; Autoclave;
|
ethanol
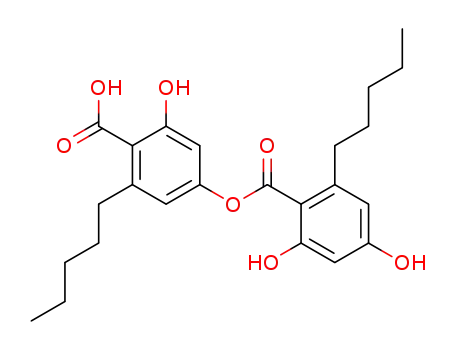
anziaic acid

3,5-dibromo-2,4-dihydroxy-6-n-pentylbenzoic acid ethyl ester
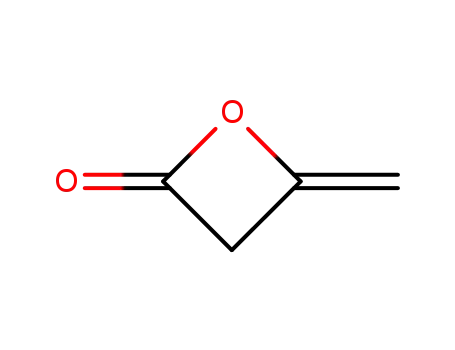
4-methyleneoxetan-2-one
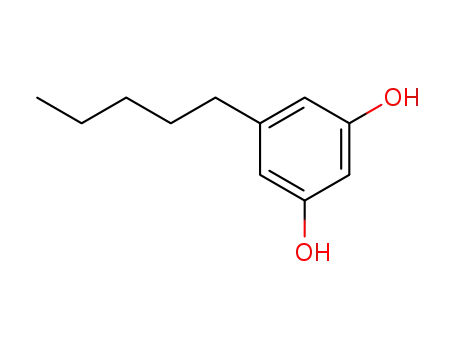
Olivetol
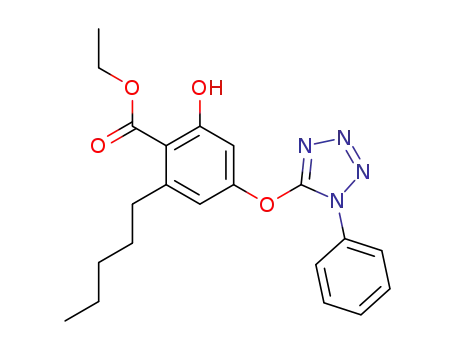
ethyl 2-hydroxy-6-pentyl-4-(1-phenyl-5-tetrazolyl)-oxybenzoate
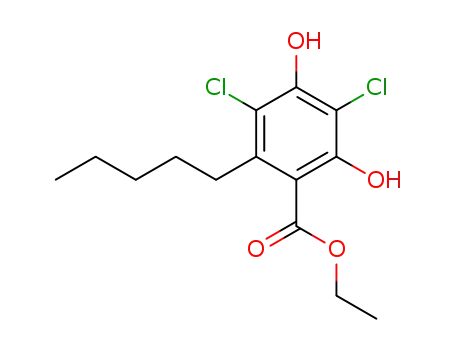
ethyl 3,5-dichloro-2,4-dihydroxy-6-pentylbenzoate
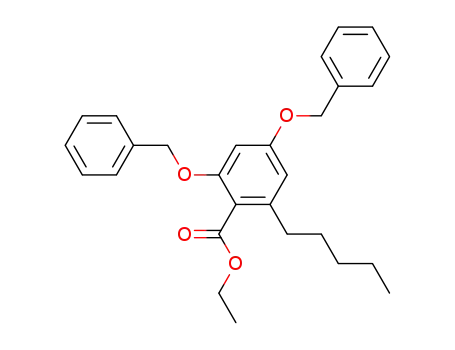
ethyl 2,4-dibenzyloxy-6-pentylbenzoate