Your Location:Home > Products > Cannabis > Heptylresorcinol acid
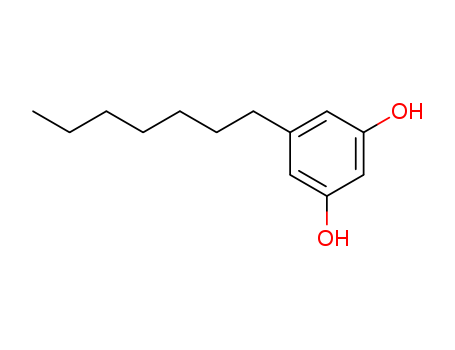

CasNo: 500-67-4
Molecular Formula: C13H20O2
|
500-67-4 Name |
|
|
Name |
Heptylresorcinol acid |
|
Synonym |
SPHEROPHORAL;1,3-Benzenediol, 5-heptyl-;5-Heptyl-1,3-benzenediol;5-HEPTYLRESORCINOL;5-Heptylbenzene-1,3-diol;5-HEPTYLRESORCINOL 95%;spherophorol;3,5-Dihydroxyheptylbenzene |
|
500-67-4 Chemical & Physical Properties |
|
|
Melting point |
55-57°C |
|
Boiling point |
342.3ºC at 760 mmHg |
|
Density |
1.033 g/cm3 |
|
Molecular Formula |
C13H20O2 |
|
Molecular Weight |
208.29700 |
|
PSA |
40.46000 |
|
LogP |
3.61070 |
|
Exact Mass |
208.14600 |
|
Index of Refraction |
1.534 |
Heptylresorcinol acid is a natural product found in Ardisia virens with data available. The chemical formula of 5-Heptylresorcinol is C13H20O2 which containing 13 Carbon atoms,20 Hydrogen atoms and 2 Oxygen atoms, and the molecular weight of 5-Heptylresorcinol is 208.301. It is used as an antiseptic and disinfectant in topical pharmaceutical products in the treatment of skin disorders and infections. 5-Heptylresorcinol and other alkylhydroxybenzenes have been used to study the protective effect in Saccharomyces cerevisiae against oxidative and radiation-caused damage.
InChI:InChI=1/C13H20O2/c1-2-3-4-5-6-7-11-8-12(14)10-13(15)9-11/h8-10,14-15H,2-7H2,1H3
Chromatography of the resulting yellow oil over silica gel (eluted with 17-20% EtOAc-hexanes) gave 270 mg (99%) of the known Heptylresorcinol acid as a pale yellow oil. …
(?)-Cannabidiol [(?)-CBD] has recently gained prominence as a treatment for neuro-inflammation and other neurodegenerative disorders; interest is also developing in its synthetic enantiomer, (+)-CBD, which has a higher affinity to CB1/CB2 receptors than the natural stereoisomer. We have developed an inexpensive, stereoselective route to access ent-CBD derivatives using (+)-carvone as a starting material. In addition to (+)-CBD, we report the first syntheses of (+)-cannabidivarin, (+)-cannabidiphorol as well as C-6/C-8 homologues.
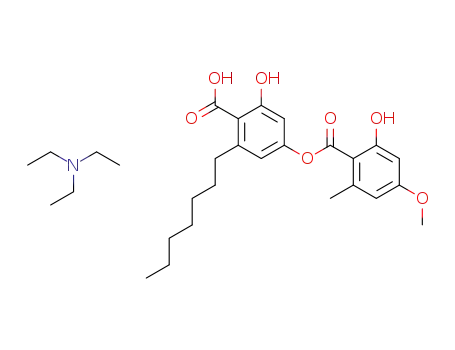
triethylammonium salt of sphaerophorin

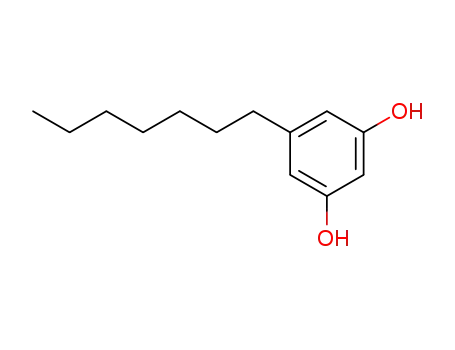
spherophorol

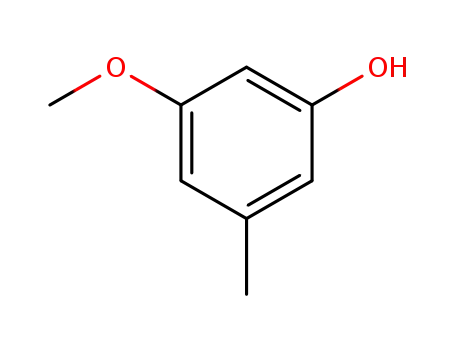
O-methylorcinol

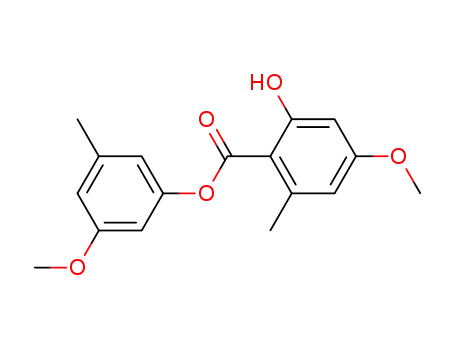
3-methoxy-5-methylphenyl 2-hydroxy-4-methoxy-6-methylbenzoate

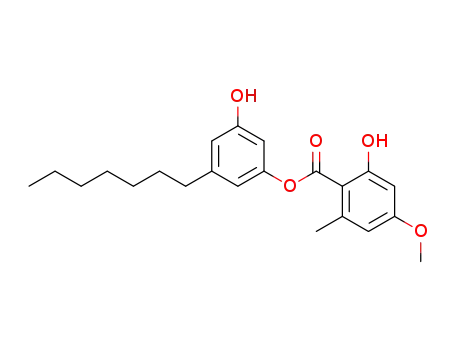
decarboxysphaerophorin
| Conditions | Yield |
|---|---|
|
at 180 ℃; for 0.166667h; Product distribution; pyrolysis;
|
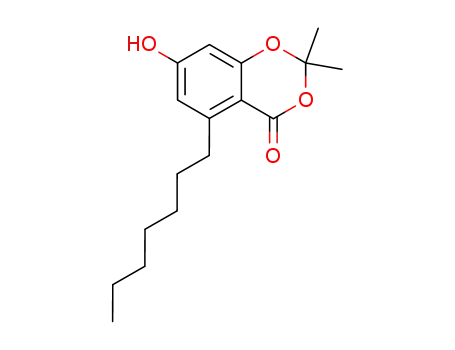
2,2-dimethyl-5-(1-heptyl)-7-hydroxy-4H-1,3-benzodioxin-4-one


spherophorol
| Conditions | Yield |
|---|---|
|
With potassium hydroxide; In dimethyl sulfoxide; at 115 ℃; for 4.5h;
|
99% |
|
With aqueous KOH; In dimethyl sulfoxide;
|
270 mg (99%) |
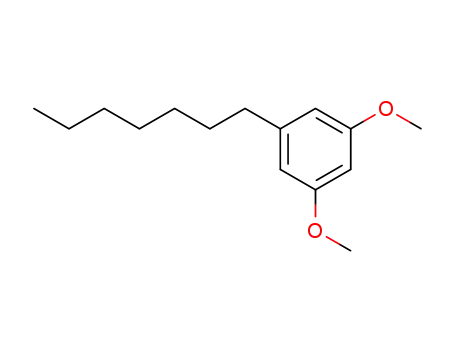
5-n-heptyl resorcinol dimethyl ether
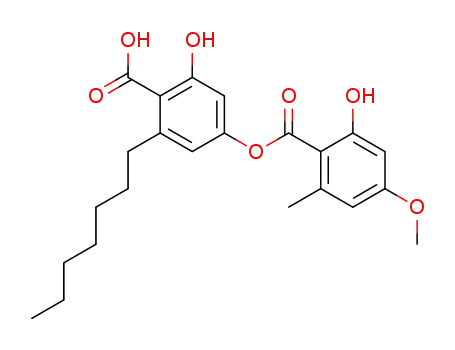
sphaerophorin
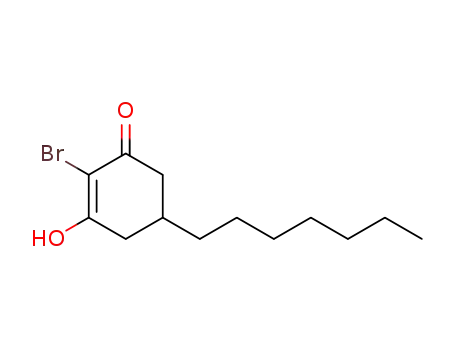
2-Bromo-5-heptyl-3-hydroxy-cyclohex-2-enone
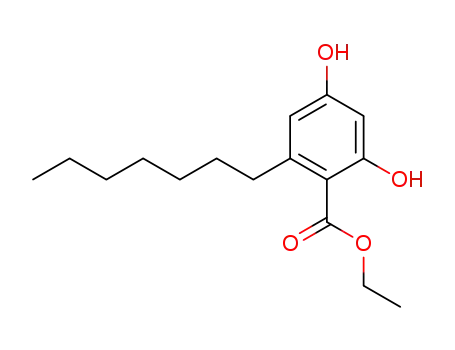
ethyl 2-heptyl-4,6-dihydroxybenzoate
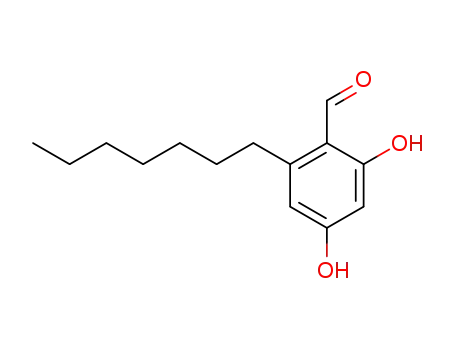
2-heptyl-4,6-dihydroxy-benzaldehyde
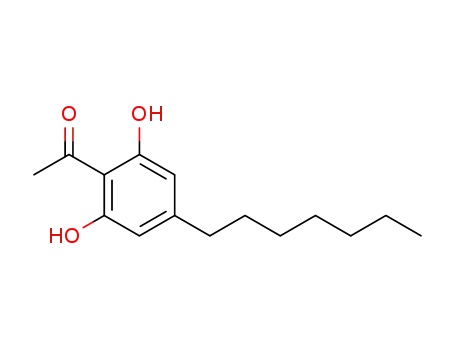
2,6-dihydroxy-4-heptylacetophenone
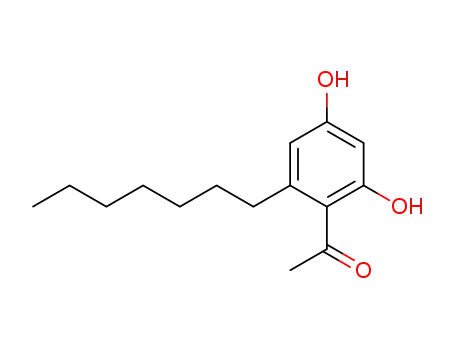
2,4-dihydroxy-6-heptylacetophenone
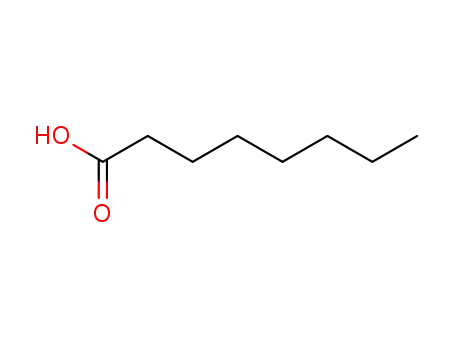
Octanoic acid