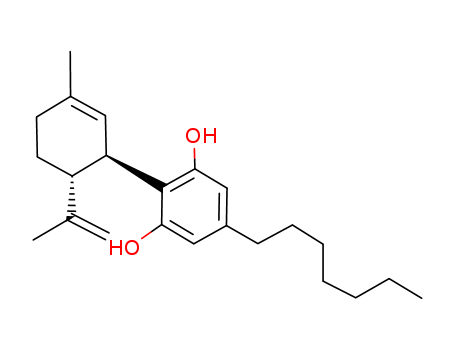

CasNo: 55824-13-0
|
55824-13-0 Name |
|
|
Name |
CBDP |
|
Synonym |
1,3-Benzenediol, 5-heptyl-2-[(1R,6R)-3-methyl-6-(1-methylethenyl)-2-cyclohexen-1-yl]-, 5-Heptyl-2-[(1R,6R)-6-isopropenyl-3-methyl-2-cyclohexen-1-yl]-1,3-benzenediol |
|
55824-13-0 Chemical & Physical Properties |
|
|
Boiling point |
488.1±45.0 °C at 760 mmHg |
|
Density |
1.0±0.1 g/cm3 |
|
Molecular Formula |
C23H34O2 |
|
Molecular Weight |
342.515 |
|
Flash Point |
212.6±23.3 °C |
|
LogP |
8.10 |
|
Exact Mass |
342.255890 |
|
Vapour Pressure |
0.0±1.3 mmHg at 25°C |
|
Index of Refraction |
1.538 |
A novel phytocannabinoid was named cannabidiphorol (CBDP) isolated from Cannabis sativa, which is a CBD homolog with a seven-term side alkyl chain. THCP and CBDP, has given the opportunity to study their distribution in cannabis germplasm.
Our interest in these latter two derivatives stems from structure activity relationship data that demonstrate the importance of the alkyl chain length and how these derivatives may bind to CB1 and CB2 receptors . Also of note, natural (−)-CBDV is in early clinical development for the treatment of autism spectrum disorders and recently, (−)-CBDP has emerged as a more potent cannabinoid than (−)-CBD itself, making it an alternative to THC therapy without the signature psychoactivity of the latter
In earlier work we have provided evidence for the presence of a subsite within the CB1 and CB2 cannabinoid receptor binding domains of classical cannabinoids. However, these affinities are significantly improved with the introduction of a C2′-C3′ cis double bond that modifies the available conformational space within the side chain and allows for a better accommodation of a six-membered ring within the side chain subsite. Our SAR results are highlighted by molecular modeling of key analogs.
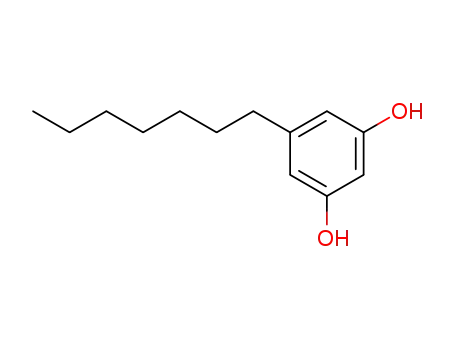
spherophorol


(1S,4R)-p-mentha-2,8-dien-1-ol

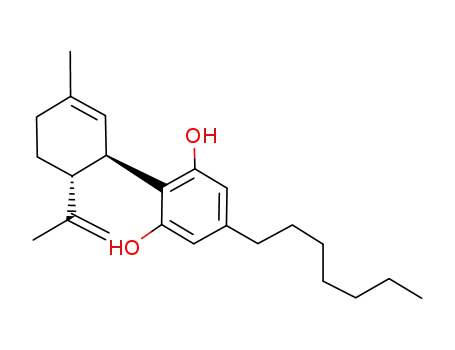
5-heptyl-2-((1R,6R)-3-methyl-6-(prop-1-en-2-yl)cyclohex-2-enyl)benzene-1,3-diol
| Conditions | Yield |
|---|---|
|
With toluene-4-sulfonic acid; In benzene; at 10 - 20 ℃;
|
32% |
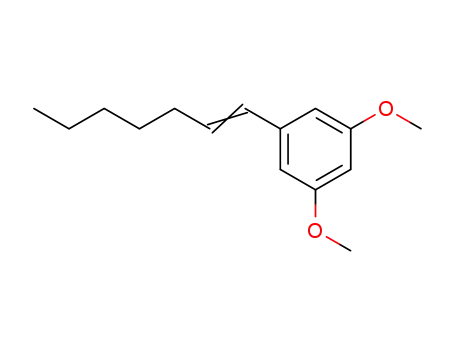
3,5-dimethoxy-1-(1-heptenyl)benzene


5-heptyl-2-((1R,6R)-3-methyl-6-(prop-1-en-2-yl)cyclohex-2-enyl)benzene-1,3-diol
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 3 steps
1: 95 percent / H2 / Pd/C / ethyl acetate / 20 °C
2: 91 percent / BBr3 / CH2Cl2 / -78 - 0 °C
3: 32 percent / p-toluenesulfonic acid / benzene / 10 - 20 °C
With hydrogen; boron tribromide; toluene-4-sulfonic acid; palladium on activated charcoal; In dichloromethane; ethyl acetate; benzene;
|

spherophorol

(1S,4R)-p-mentha-2,8-dien-1-ol

3,5-dimethoxy-1-(1-heptenyl)benzene
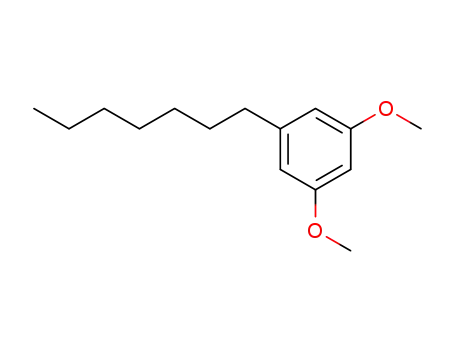
5-n-heptyl resorcinol dimethyl ether