Your Location:Home > Products > 2-Bromo-1-phenylpropane


CasNo: 2114-39-8
Molecular Formula: C9H11 Br
Appearance: clear colorless to light brown liquid
|
2114-39-8 Name |
|
|
Name |
2-Bromo-1-phenylpropane |
|
Synonym |
Benzene,(2-bromopropyl)-;2-BROMO-1-PHENYLPROPANE;(2-BROMOPROPYL)BENZENE;1-PHENYL-2-BROMO PROPANE;α-Methylphenethyl bromide;rac-(R*)-1-Phenyl-2-bromopropane;2-Bromo-1-phenylpropane,97%;2-Bromo-1-phenylpropane,98% |
|
Chemical & Physical Properties |
|
|
Melting point |
-10°C (estimate) |
|
Boiling point |
107-109 °C16 mm Hg(lit.) |
|
Density |
1.291 g/mL at 25 °C(lit.) |
|
Molecular Formula |
C9H11Br |
|
Molecular Weight |
199.08800 |
|
Flash Point |
195 °F |
|
PSA |
3.01250 |
|
Exact Mass |
198.00400 |
|
Vapour Pressure |
0.113mmHg at 25°C |
|
Index of Refraction |
n20/D 1.545(lit.) |
2-Bromo-1-phenylpropane is a useful research chemical which is a liquid that is used as an intermediate in the production of styrene. It is prepared by the reaction of ethylmagnesium bromide with arylacetylene.
Chemical Properties
clear colorless to light brown liquid
InChI:InChI=1/C9H11Br/c1-8(10)7-9-5-3-2-4-6-9/h2-6,8H,7H2,1H3
2-Bromo-1-phenylpropane is expected to exhibit all the above complications. Assuming a linear dependence of tJ* on electronegativity, 6J* can be predicted for 2-bromo-1-phenylpropane by extrapolating between the values for toluene and benzylfluoride using the electronegativity of the -CHBTCH, group.
The search for metal-free, stable and high effective photocatalysts with sufficient photo-redox potentials remains a key challenge for organic chemists. Here, we present a benzothiadiazole-containing molecular organic photocatalyst with redox potentials of ?1.30 V and +1.64 V vs. SCE. Lastly, no photo-bleaching effect is observed, demonstrating the high stability and recyclable of the designed organic photocatalyst. (Figure presented.).
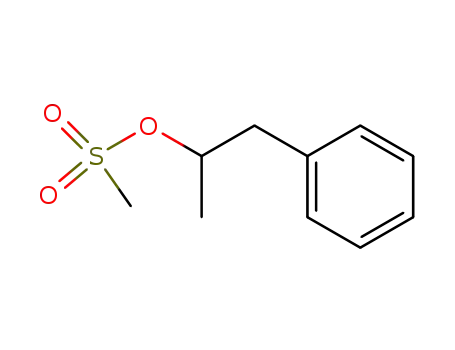
(±)-1-phenylpropanyl-2-yl methansulfonate

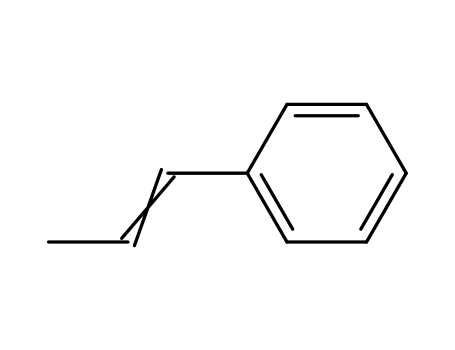
1-phenylpropene

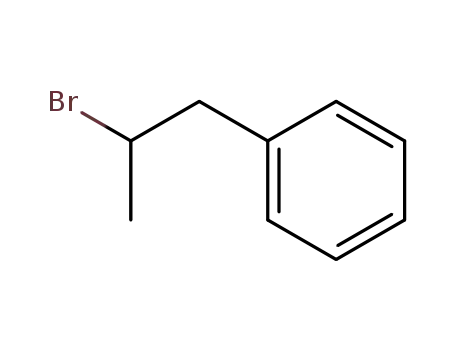
(2-bromopropyl)-benzene
| Conditions | Yield |
|---|---|
|
With 1-n-butyl-3-methylimidazolim bromide; at 90 ℃; for 1h; Concentration; Temperature; Time; Inert atmosphere; Green chemistry;
|
95% 34% |
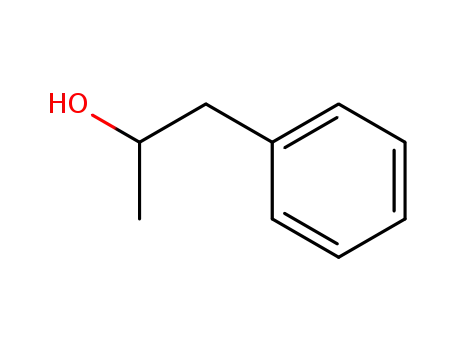
3-phenyl-2-propanol


1-phenylpropene

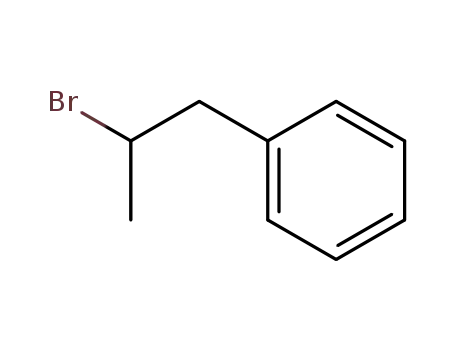
(2-bromopropyl)-benzene
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1: pyridine / 20 °C
2: 1-n-butyl-3-methylimidazolim bromide / 1 h / 90 °C / Inert atmosphere; Green chemistry
With pyridine; 1-n-butyl-3-methylimidazolim bromide;
|
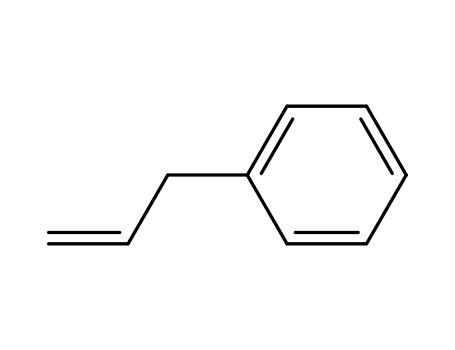
allylbenzene

3-phenyl-2-propanol
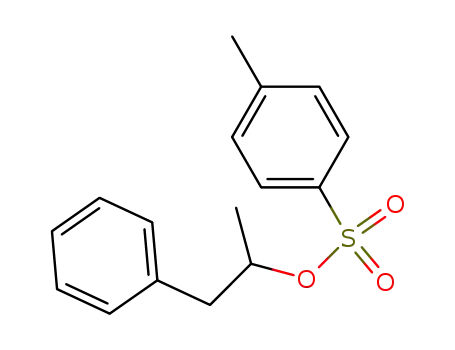
1-phenyl-2-propyl-4-toluenesulfonate
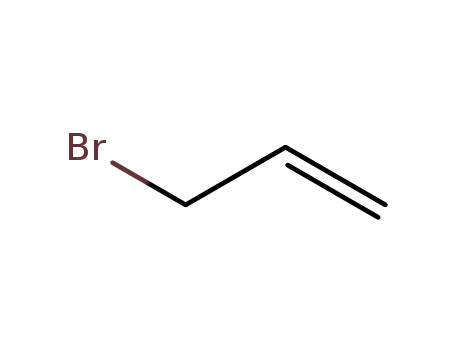
allyl bromide
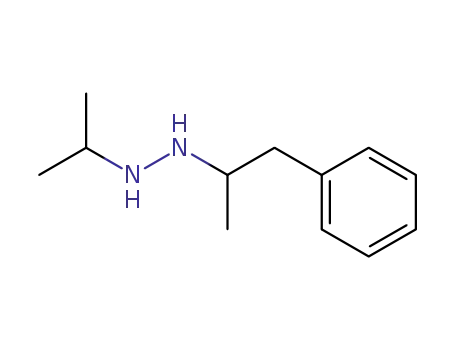
N-(Phenylisopropyl)-N'-isopropylhydrazin
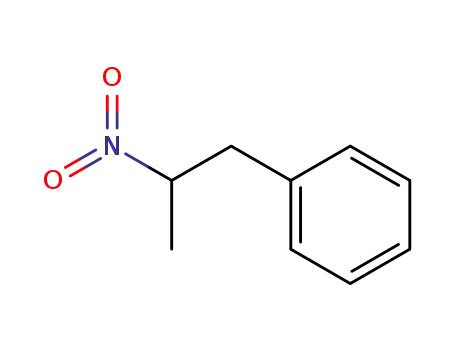
(2-nitropropyl)benzene
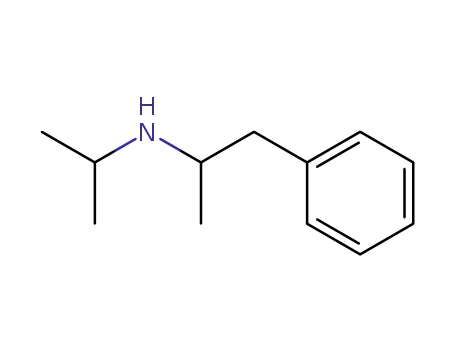
N-isopropyl-2-amino-1-phenylpropane
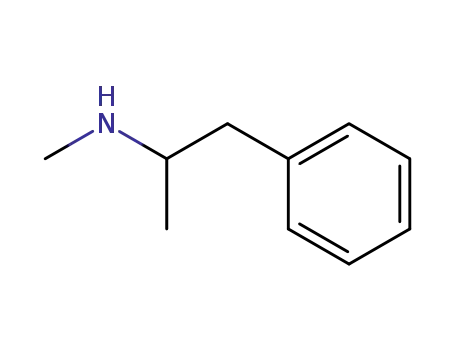
methamphetamin