Your Location:Home > Products > Cannabis > Tetrahydrocannabiphorol

CasNo: 54763-99-4
Molecular Formula: C23H34 O2
|
54763-99-4 Name |
|
|
Name |
Tetrahydrocannabiphorol |
|
Synonym |
3-heptyl-delta(1)-tetrahydrocannabinol;6H-Dibenzo[b,d]pyran-1-ol, 3-heptyl-6a,7,8,10a-tetrahydro-6,6,9-trimethyl-, (6aR-trans)- (9CI);Δ9-THCP;Tetrahydrocannabiphorol (THCP) |
|
54763-99-4 Chemical & Physical Properties |
|
|
Molecular Formula |
C23H34O2 |
|
Molecular Weight |
342.51500 |
|
PSA |
29.46000 |
|
LogP |
6.51600 |
|
Exact Mass |
342.25600 |
Tetrahydrocannabiphorol (THCP) is a potent phytocannabinoid, a CB1 and CB2 agonist which was known as a synthetic homologue of THC. THCP, which is short for tetrahydrocannabiphorol and scientifically known as (-)-Trans-Δ9-tetrahydrocannabiphorol, is a natural cannabinoid found in the cannabis plant but in super low traces. It takes the #1 spot for the strongest natural cannabinoid ever discovered.
InChI:InChI=1/C23H34O2/c1-5-6-7-8-9-10-17-14-20(24)22-18-13-16(2)11-12-19(18)23(3,4)25-21(22)15-17/h13-15,18-19,24H,5-12H2,1-4H3/t18-,19-/m1/s1
The (−)-trans-Δ9-tetrahydrocannabiphorol (Δ9-THCP, 1) content of the inflorescence from six Cannabis sativa chemotypes, including 14 plants of distinct genotypes, and two extracts was determined quantitatively via high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS). (−)-trans-Δ9‑Tetrahydrocannabiphorol Content of Cannabis sativa Inflorescence from Various Chemotypes.
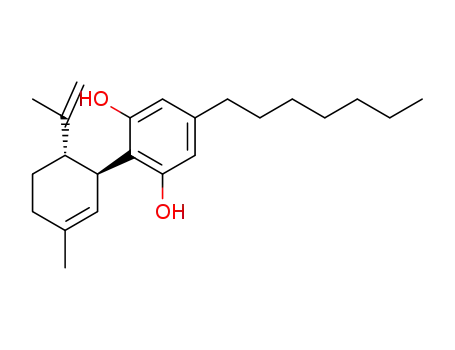
5-heptyl-2-((1S,6S)-3-methyl-6-(prop-1-en-2-yl)cyclohex-2-enyl)benzene-1,3-diol

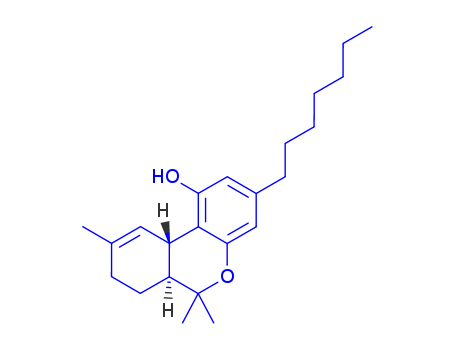
![(6aS,10aS)-3-heptyl-6,6,9-trimethyl-6a,7,8,10a-tetrahydro-6H-benzo[c]chromen-1-ol](/upload/2023/1/b8c1fc9b-bf37-47e3-96fb-d6179f958c31.png)
(6aS,10aS)-3-heptyl-6,6,9-trimethyl-6a,7,8,10a-tetrahydro-6H-benzo[c]chromen-1-ol
| Conditions | Yield |
|---|---|
|
With triisobutylaluminum; In hexane; dichloromethane; at 20 ℃; for 24h; Inert atmosphere;
|
![(1′R,2′R)-5′-methyl-2′-(prop-1-en-2-yl)-1′,2′,3′,4′-tetrahydro-[1,1′-biphenyl]-2,4,6-triol](/upload/2023/1/dd4479e4-12b7-4ebc-a7ad-12081a1f642d.png)
(1′R,2′R)-5′-methyl-2′-(prop-1-en-2-yl)-1′,2′,3′,4′-tetrahydro-[1,1′-biphenyl]-2,4,6-triol

![(6aS,10aS)-3-heptyl-6,6,9-trimethyl-6a,7,8,10a-tetrahydro-6H-benzo[c]chromen-1-ol](/upload/2023/1/b8c1fc9b-bf37-47e3-96fb-d6179f958c31.png)
(6aS,10aS)-3-heptyl-6,6,9-trimethyl-6a,7,8,10a-tetrahydro-6H-benzo[c]chromen-1-ol
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 4 steps
1.1: triethylamine / dichloromethane / 0 - 20 °C / Inert atmosphere
2.1: triethylamine / dichloromethane / 0 - 20 °C / Inert atmosphere
3.1: zinc dibromide; lithium bromide / diethyl ether; tetrahydrofuran / 0.5 h / Inert atmosphere
3.2: 6 h / 20 °C / Inert atmosphere
4.1: triisobutylaluminum / dichloromethane; hexane / 24 h / 20 °C / Inert atmosphere
With triisobutylaluminum; triethylamine; lithium bromide; zinc dibromide; In tetrahydrofuran; diethyl ether; hexane; dichloromethane;
|
|
|
Multi-step reaction with 4 steps
1.1: triethylamine / dichloromethane / 20 °C / Inert atmosphere
2.1: triethylamine / dichloromethane / 0 - 20 °C / Inert atmosphere
3.1: zinc dibromide; lithium bromide / diethyl ether; tetrahydrofuran / 0.5 h / Inert atmosphere
3.2: 6 h / 20 °C / Inert atmosphere
4.1: triisobutylaluminum / dichloromethane; hexane / 24 h / 20 °C / Inert atmosphere
With triisobutylaluminum; triethylamine; lithium bromide; zinc dibromide; In tetrahydrofuran; diethyl ether; hexane; dichloromethane;
|
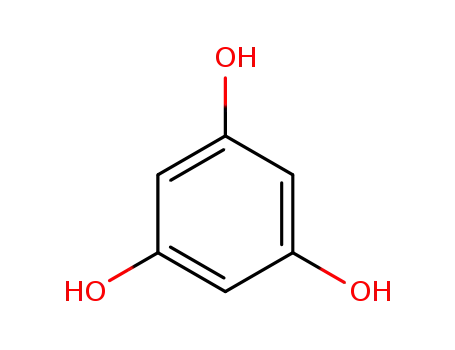
3,5-dihydroxyphenol

(1S,4R)-p-mentha-2,8-dien-1-ol