Your Location:Home > Products > Fine Chemicals > DEDC
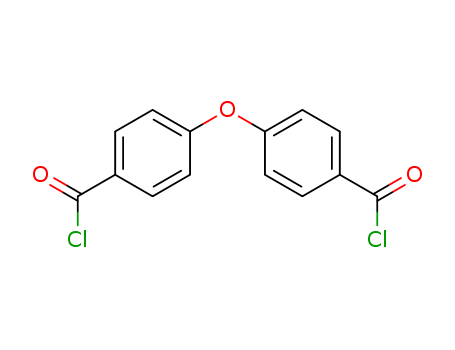

CasNo: 7158-32-9
Molecular Formula: C14H8 Cl2 O3
|
7158-32-9 Name |
|
|
Name |
DEDC |
|
Synonym |
4,4'-OXYBISBENZOYL CHLORIDE,4.4-oxybisbenzoic chloride;4-(4-carbonochloridoylphenoxy)benzoyl chloride;4,4-Dicarboxylic acid diphenyl oxide dichloroanhydride;p,p'-Oxybis(benzoyl chloride);4,4'-OXYBISBENZOYL CHLORIDE;4,4-OXY-DI-BENZOYLCHLORIDE;4,4'-Oxybisbenzoyl chloride(DEDC/ODBC);4-(Chlorocarbonyl)phenyl ether |
|
7158-32-9 Chemical & Physical Properties |
|
|
Melting point |
22-23ºC |
|
Boiling point |
403.7±30.0 °C at 760 mmHg |
|
Density |
1.4±0.1 g/cm3 |
|
Molecular Formula |
C14H8Cl2O3 |
|
Molecular Weight |
295.117 |
|
Flash Point |
164.1±23.6 °C |
|
PSA |
43.37000 |
|
LogP |
4.69 |
|
Exact Mass |
293.985046 |
|
Vapour Pressure |
0.0±0.9 mmHg at 25°C |
|
Index of Refraction |
1.604 |
|
7158-32-9 Uses |
|
4,4'-OXYBISBENZOYL CHLORIDE is a useful research chemical. |
Physical Form
Crystal - Powder
Uses
4,4'-OXYBISBENZOYL CHLORIDE is a useful research chemical.
A new rod-like thermotropic liquid crystalline polyester (TLCP) material and its nanocomposites based on different concentrations of graphene were synthesized by in-situ high-temperature solution polymerization. The resulting nanocomposites were characterized using XRD, microscopic analysis (POM, SEM, and TEM), spectroscopic analysis (FT-IR, UV-Vis, and fluorescence), and thermal analysis (TGA and DSC). The XRD and POM methods showed that the composite materials exhibited only the nematic phase. The TEM images revealed that the graphene were distributed in the polymer with sizes ranging from 100 to 200 nm. The absorption spectroscopy data showed that the electronic properties of graphene were mostly retained without damaging their two-dimensional electronic properties, together with the analysis of the maximum absorption spectrum and concentration of the composites in terms of the Lambert-Beer law. The fluorescence from the TLCP moiety was almost completely quenched and red shifted by graphene, indicating that the linkage mode facilitated effective energy and electron transfer between the rod-like TLCP and the extended .-system of graphene. Therefore, this novel nanocomposite material exhibits excellent thermal properties based on the thermogravimetric analysis. Copyright
Recently, scientists have reported a range of chiral fluorescence materials or chiral composites that can emit circularly polarized luminescence. Herein, two achiral metal–organic colloidal solutions were studied, showing active circularly polarized luminescence, which is observed in vortex stirring. The absolute values for glum are 0.05 and 0.03 and the plus or minus sign of glum depends on the colloidal structure and stirring direction, which make the property easy to manipulate. Further, the host–guest interaction study suggests both electrostatic interactions and coordination bonding may influence the chiroptical property from the colloidal solution to the guest molecule. Rhodamine 6G and its carboxylic acid derivative exhibit good quantum yields and acceptable glum values in the colloidal solution.
Zn(II) and Cd(II) coordination polymers constructed from a new angular bis-pyridyl-bis-amide ligand and naphthalene-1,4-dicarboxylic acid (1,4-H2NDC), {[Zn2(L)(1,4-NDC)2]·2H2O} [L = 4,4′-oxybis(N-(pyridin-3-ylmethyl)benzamide)], 1, and {[Cd2(L)(1,4-NDC)2]·EtOH}, 2, have been solvothermally synthesized and structurally characterized by using single-crystal X-ray diffraction. While complex 1 displays a 2D double layer with the rare (46·64)-5 L4 topology, complex 2 shows a 3D framework with a new (4·5·6)(45·55·69·7·8) topology. Both complexes 1 and 2 display luminescence properties and 2 exhibits a high sensitivity toward the detection of Fe3+ ions with a Ksv value of 1.9 × 104 M-1. The quenching effect can most probably be ascribed to the interactions between the metal ions and the amide carbonyl oxygen atoms.
Abstract: New 4,4′-oxydibenzamides and sulfamoyl derivatives of 4,4′-oxydibenzoic acid containing pharmacophoric 2-arylaminopyrimidine fragments in the amide moiety have been synthesized. Acylation of amines of the pyrimidine series with 2-chlorosulfonyl-substituted 4,4′-oxydibenzoic acid gave the corresponding sulfonamides. Arylaminopyrimidine derivatives of 4,4′-oxydibenzamide were obtained in 75–87% yield by acylation of 3-{[4-(pyridin-3-yl)pyrimidin-2-yl]amino}anilines with 4,4′-oxydibenzoyl chloride. The reactivity of 4,4′-oxydibenzoyl chloride toward amines is discussed.
The invention discloses an (octylphenol polyoxyethylene ether disubstituted) dicarboxylic diphenyl ether nonionic gemini surfactant and synthesis thereof. The (octylphenol polyoxyethylene ether disubstituted) dicarboxylic diphenyl ether nonionic gemini surfactant adopts a structural formula as follows: FORMULA, and is prepared through a two-step reaction as follows: S1, performing an acyl chlorination reaction on 4,4'-dicarboxylic diphenyl ether to obtain 4,4'-diformyl chloride diphenyl ether; S2, performing an esterification reaction on the 4,4'-diformyl chloride diphenyl ether and octylphenol polyoxyethylene ether (OP-10) to obtain a target product (octylphenol polyoxyethylene ether disubstituted) dicarboxylic diphenyl ether. The surfactant is expected to be applied to alkali/surfactantand polymer/surfactant binary compound flooding fluids and an alkali/surfactant/polymer ternary compound flooding fluid, a microemulsion emulsifier and the like in tertiary oil recovery and can be also compounded with a common surfactant to reduce the using cost, so that a condition is created for scale application thereof.
4-(4-formylphenoxy)benzaldehyde

4,4'-bis(chlorocarbonyl)diphenyl oxide
| Conditions | Yield |
|---|---|
|
With thionyl chloride;
|
4-(4'-cyanophenoxy)benzonitrile


4,4'-bis(chlorocarbonyl)diphenyl oxide
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1: aqueous H2SO4
2: SOCl2
With thionyl chloride; sulfuric acid;
|
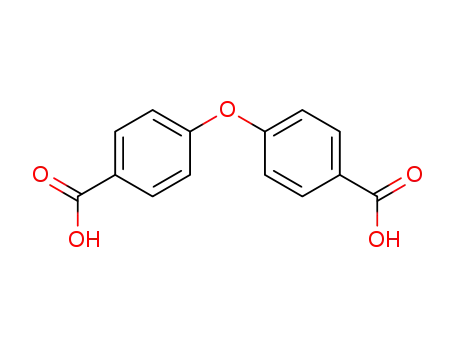
oxybis(benzoic acid)

4-(4-formylphenoxy)benzaldehyde

4-(4'-cyanophenoxy)benzonitrile
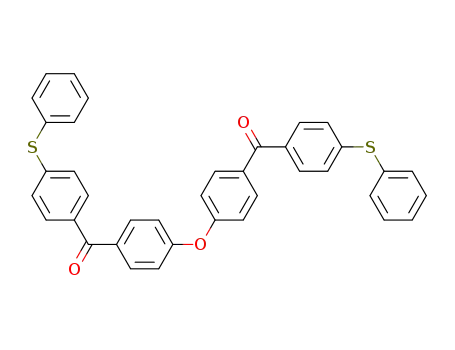
(4'-phenylthio-4-benzoylphenyl) ether

4-(4-formylphenoxy)benzaldehyde
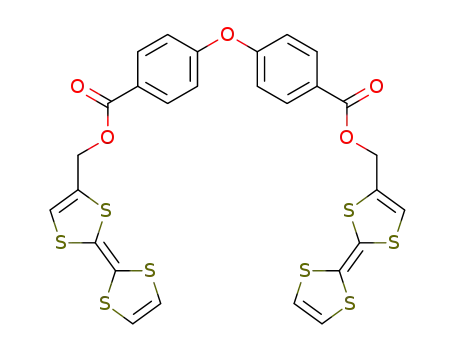
bis(tetrathiafulvalen-4-ylmethyl) 4,4'-oxybis(benzenecarboxylate)
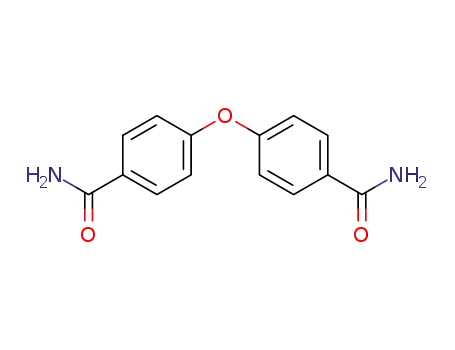
4-(4-carbamoylphenoxyl)benzamide