Your Location:Home > Products > Fine Chemicals > ATP-Na2, Adenosine 5'-triphosphate disodium salt
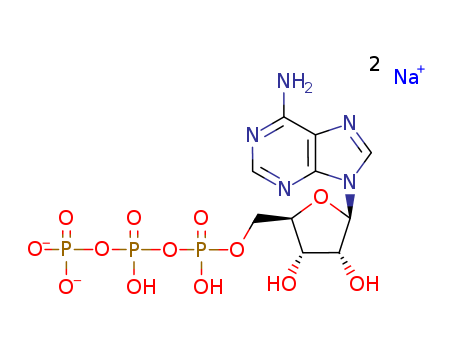

CasNo: 987-65-5
Molecular Formula: C10H14N5Na2O13P3
Appearance: Solid.
|
987-65-5 Name |
|
|
Name |
ATP-Na2 |
|
Synonym |
Adenosine 5’-triphosphate disodium salt,ADENOSINE TRIPHOSPHATE DISODIUM;ADENOSINE TRIPHOSPHATE, DISODIUM SALT;ADENYLPYROPHOSPHORIC ACID DISODIUM SALT;ADENOSINE-5'-TRIPHOSPHATE HYDRATE DISODIUM SALT;ADENOSINE-5'-TRIPHOSPHATE NA2-SALT;ADENOSINE-5'-TRIPHOSPHORIC ACID, DISODIUM;ADENOSINE-5'-TRIPHOSPHORIC ACID DISODIUM DIHYDROGEN SALT;ADENOSINE 5'-TRIPHOSPHORIC ACID DISODIUM SALT |
|
987-65-5 Biological Activity |
|
|
Related Catalog |
Signaling Pathways >> Membrane Transporter/Ion Channel >> P2X Receptor Natural Products >> Alkaloid Research Areas >> Metabolic Disease |
|
987-65-5 Chemical & Physical Properties |
|
|
Melting point |
188-190ºC |
|
Boiling point |
951.4ºC at 760mmHg |
|
Density |
2.63g/cm3 |
|
Molecular Formula |
C10H14N5Na2O13P3 |
|
Molecular Weight |
551.145 |
|
PSA |
314.22000 |
|
Exact Mass |
550.959656 |
|
Water Solubility |
H2O: 50 mg/mL |
Adenosine 5'-triphosphate disodium salt is a disodium salt form of adenosine-triphosphate which is a multifunctional nucleoside triphosphate. It is a multifunctional nucleoside triphosphate used in cells as a coenzyme of intracellular energy transfer. It transports chemical energy within cells for metabolism. ATP Disodium Salt is used in the synthetic preparation of ribose-modified deoxyadenosine bisphosphate analogues as P2Y1 receptor ligands. In some studies, adenosine 5'-triphosphate disodium salt hydrate (ATP-Na2) was used on the basis of the CI inhibition.
The present study compared the effects of ATP-MgCl2 or ATP-Na2 administration on renal function and cellular metabolism following renal ischemia in rabbits. These results indicate that ATP should be given in the form of ATP-MgCl2 for it to be effective following renal ischemia. The results also suggest that the salutary effect of ATP-MgCl2 following renal ischemia could occur through the improvement of cellular metabolism and concomitant improvement in tissue blood flow.
A general and high-yielding synthesis of nucleoside 5′-triphosphates (NTPs) and nucleoside 5′-diphosphates (NDPs) from protected nucleoside 5′-phosphoropiperidates promoted by 4,5-dicyanoimidazole (DCI) has been developed. The experimental results suggested that the mechanism of DCI activation could be distinctive for NTP and NDP synthesis, depending on the different nucleophilicity of pyrophosphate and phosphate.
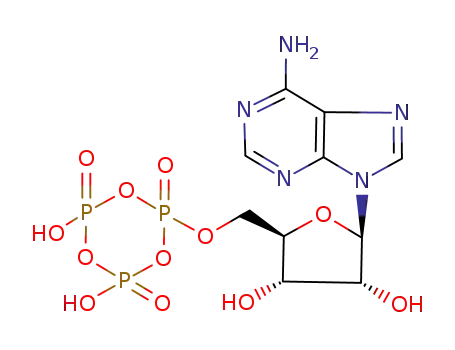
adenosine 5'-trimetaphosphate

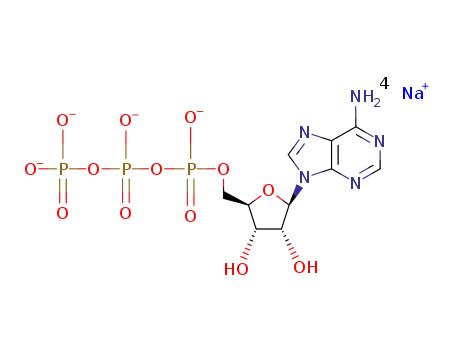
adenosine 5'-triphosphate

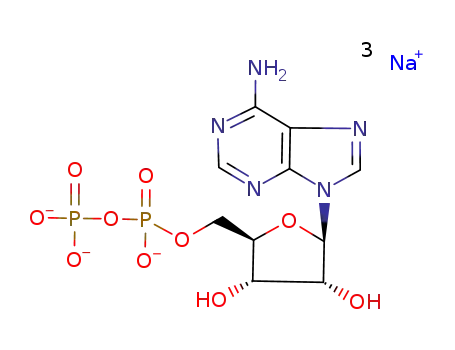
adenosine-5′-diphosphate trisodium salt
| Conditions | Yield |
|---|---|
|
Yield given. Multistep reaction. Yields of byproduct given. Title compound not separated from byproducts;
|
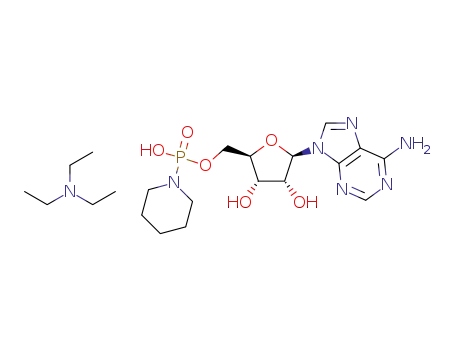
adenosine 5′-phosphoropiperidate triethylammonium salt


adenosine 5'-triphosphate
| Conditions | Yield |
|---|---|
|
adenosine 5′-phosphoropiperidate triethylammonium salt; With 4,5-dicyano-1H-imidazole; tris(tetra-n-butylammonium) hydrogen pyrophosphate; In N,N-dimethyl-formamide; at 20 ℃; for 6h; Inert atmosphere;
With sodium acetate; In ethanol; water; Further stages; Inert atmosphere;
|
77% |

adenosine 5'-trimetaphosphate
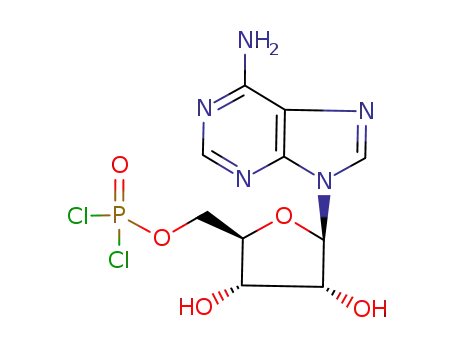
C10H12Cl2N5O5P
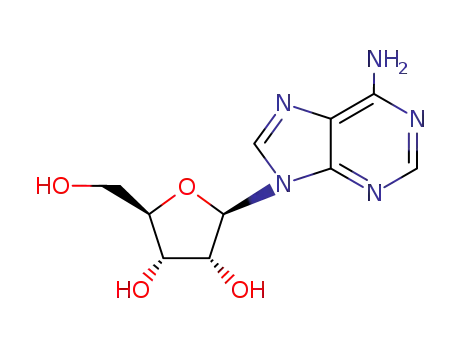
adenosine
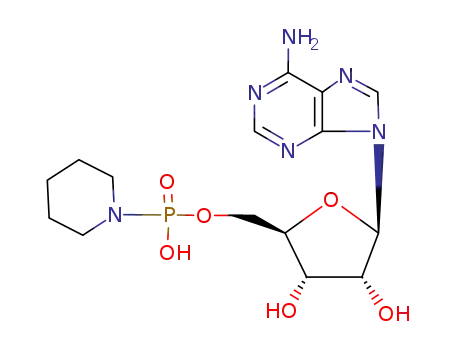
adenosine (5'-phosphoro-1-piperidinide)
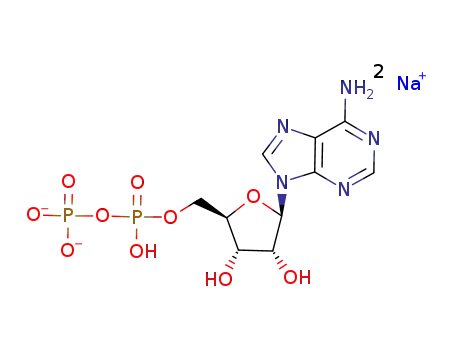
adenosine diphosphate disodium salt
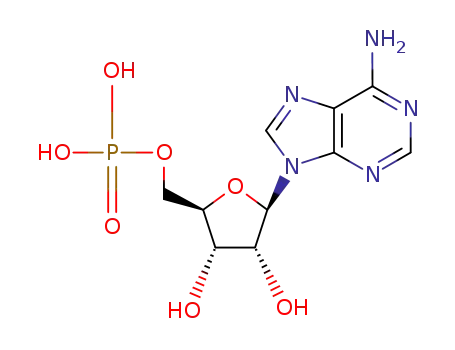
5'-adenosine monophosphate
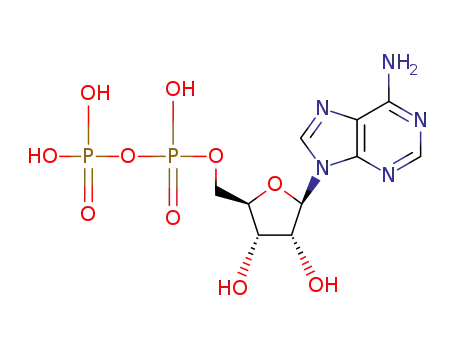
adenosine 5'-diphosphate