Your Location:Home > Products > 4-Benzo[b]thien-2-ylbenzenamine
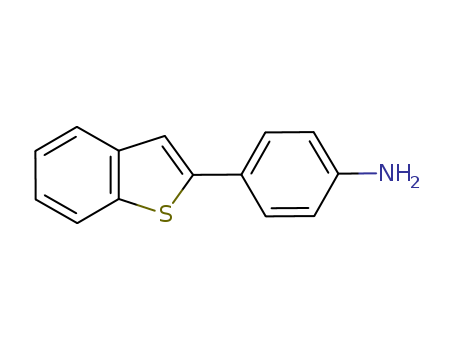

CasNo: 54492-95-4
Molecular Formula: C14H11NS
|
54492-95-4 Name |
|
|
Name |
4-Benzo[b]thien-2-ylbenzenamine |
|
Synonym |
Benzenamine, 4-benzo[b]thien-2-yl-;4-Benzo[b]thien-2-ylbenzenamine |
|
Chemical & Physical Properties |
|
|
Molecular Formula |
C14H11NS |
|
Molecular Weight |
225.31 |
|
InChI |
The Key: KCZKJUGTTGALLE-UHFFFAOYSA-N |
FLT3 inhibitors have been explored as a viable therapy for acute myeloid leukemia (AML). However, the clinical outcomes of these FLT3 inhibitors were underwhelming except AC220. Therefore, the development of novel FLT3 inhibitors with high potency against
Novel heterocyclic amide derivatives having pharmacological effects, that is, compounds represented by the general formula (1) or salts thereof: (1) wherein X1-X2 is S-CH2 or the like; R1 is alkyl or the like; p is 0 to 7; R2 is hydrogen, alkyl, or the like; R3 is hydrogen, alkyl, or the like; Y1-Y2 is CH=CH or the like; R4 is halogeno, alkyl, or the like; q is 0 to 4; and R5 is halogeno, hydrogen, alkyl, or the like.
![benzo[b]thiophene-2-boronic acid](/upload/2023/1/16eab3dd-2ef6-44ed-a623-89463aaa65a4.png)
benzo[b]thiophene-2-boronic acid

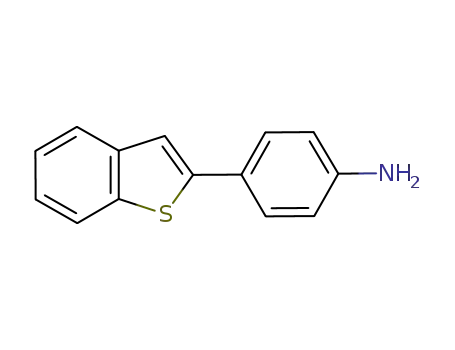
2-(4-aminophenyl)benzothiophene
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1: sodium phosphate dodecahydrate; palladium 10% on activated carbon / water; isopropyl alcohol / 4 h / 80 °C / Inert atmosphere
2: iron; ammonium chloride / ethanol; water / 1 h / Reflux
With sodium phosphate dodecahydrate; palladium 10% on activated carbon; iron; ammonium chloride; In ethanol; water; isopropyl alcohol; 1: |Suzuki Coupling;
|
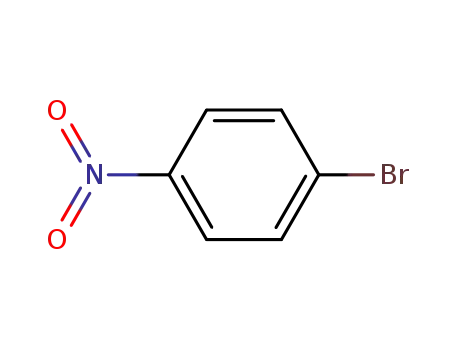
para-nitrophenyl bromide


2-(4-aminophenyl)benzothiophene
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1: sodium phosphate dodecahydrate; palladium 10% on activated carbon / water; isopropyl alcohol / 4 h / 80 °C / Inert atmosphere
2: iron; ammonium chloride / ethanol; water / 1 h / Reflux
With sodium phosphate dodecahydrate; palladium 10% on activated carbon; iron; ammonium chloride; In ethanol; water; isopropyl alcohol; 1: |Suzuki Coupling;
|
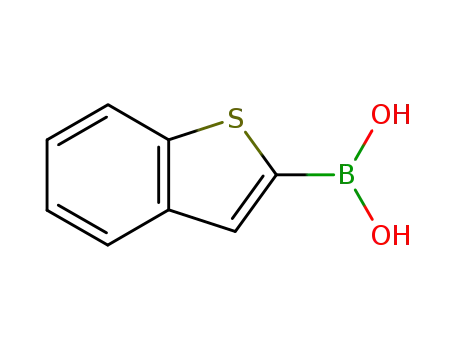
benzo[b]thiophene-2-boronic acid

para-nitrophenyl bromide