Your Location:Home > Products > Fine Chemicals > Hydroxylamine,O-(diphenylphosphinyl)-
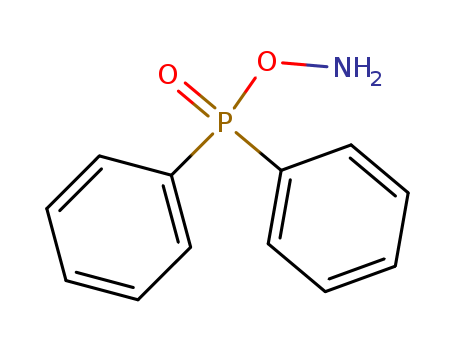

CasNo: 72804-96-7
Molecular Formula: C12H12 N O2 P
|
72804-96-7 Name |
|
|
Name |
O-DIPHENYLPHOSPHINYLHYDROXYLAMINE |
|
Synonym |
O-DIPHENYLPHOSPHINYLHYDROXYLAMINE;O-(diphenylphosphoryl)hydroxylaMine;TwoPhenylphosphonichydroxylaMine;diphenylphosphinate;O-(Diphenylphosphinyl)hydroxylamine;aMino diphenylphosphinate;(AMinooxy)diphenylphosphine oxide;HydroxylaMine, O-(diphenylphosphinyl)- |
|
72804-96-7 Chemical & Physical Properties |
|
|
Melting point |
>140℃ (decomposition) |
|
Boiling point |
368.0±25.0 °C at 760 mmHg |
|
Density |
1.25±0.1 g/cm3 |
|
Molecular Formula |
C12H12NO2P |
|
Molecular Weight |
233.203 |
|
Flash Point |
176.3±23.2 °C |
|
PSA |
62.13000 |
|
LogP |
1.68 |
|
Exact Mass |
233.060562 |
|
Vapour Pressure |
0.0±0.8 mmHg at 25°C |
|
Index of Refraction |
1.597 |
|
Storage condition |
2-8°C |
|
Water Solubility |
Slightly soluble (2.1 g/L) (25 ºC) |
O-(Diphenylphosphinyl)hydroxylamine ((ODPH)) is a general reagent for electrophilic C-amination, which has been used to aminate imides such as phthalimide in high yield. Phosphines can be aminated by reaction with O-diphenylphosphinylhydroxylamine.
A critical evaluation is presented of the scope of amination reactions with O-diphenylphosphinylhydroxylamine (ODPH) as compared to those using hydroxylamine-O-sulfonic acid (HOSA). In the early eighties a phosphorus analog of 1, theO-diphenylphosphinylhydroxylamine (2, ODPH) was proposed [4—8] as an alternative reagent for the amination of carbon, nitrogen, sulfur and phosphorus nuclei.
According to the preparation method of the O-diphenyl phosphoryl hydroxylamine compound disclosed by the embodiment of the invention, Cbz-hydroxylamine with a protecting group is used as a raw material to react with diphenyl phosphinyl chloride, so that the selectivity of O-phosphonyl protection is improved, and the production cost is reduced; the whole operation is carried out under an alkaline condition, the stability of oxygen-phosphorus bonds can be maintained, and the generation of diphenyl hypophosphorous acid byproducts is avoided, so that the intermediate can be directly used for the next step of reaction.
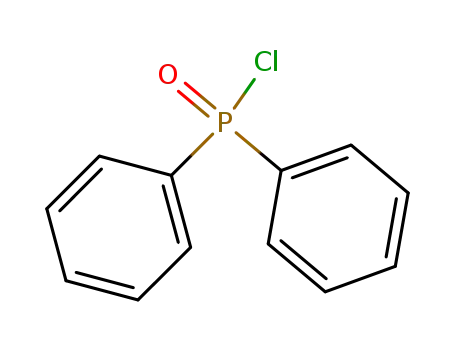
Diphenylphosphinic chloride

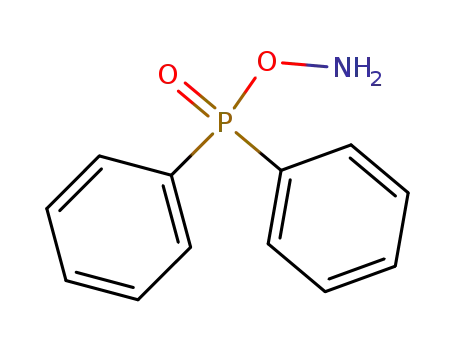
O-(diphenylphosphinyl)hydroxylamine
| Conditions | Yield |
|---|---|
|
With sodium hydroxide; hydroxylamine hydrochloride; In 1,4-dioxane; water; at 0 - 22 ℃; for 0.166667h;
|
82% |
|
Diphenylphosphinic chloride; With hydroxylamine hydrochloride; sodium hydroxide; In water; at -15 - 0 ℃; for 0.416667h;
With sodium hydroxide; In water; at 0 ℃; for 0.5h;
|
78% |
|
With hydroxylamine hydrochloride; triethylamine; In dichloromethane; at -20 - 20 ℃;
|
75% |
|
With hydroxylamine; sodium hydrogencarbonate; In dichloromethane; 1.) -30 deg C, 2 h, 2.) 20 deg C, 2 h;
|
71% |
|
With sodium hydroxide; hydroxylamine hydrochloride; In 1,4-dioxane; water; cooling;
|
70% |
|
With sodium hydroxide; hydroxylamine hydrochloride; In 1,4-dioxane; at -8 ℃; for 0.0833333h;
|
66% |
|
With hydroxylamine hydrochloride; In 1,4-dioxane; water; for 0.0666667h;
|
61% |
|
With hydroxylamine hydrochloride; sodium hydroxide; In 1,4-dioxane; water; at -5 ℃; for 0.25h;
|
61% |
|
With hydroxylamine hydrochloride; sodium hydroxide; In 1,4-dioxane; water; for 0.0833333h; Cooling with ice-salt bath;
|
59% |
|
With hydroxylamine hydrochloride; N-ethyl-N,N-diisopropylamine; In dichloromethane; at 0 - 30 ℃; for 2h; Inert atmosphere;
|
56% |
|
With hydroxylamine hydrochloride; N-ethyl-N,N-diisopropylamine; In dichloromethane; at 0 - 30 ℃; for 2h; Inert atmosphere;
|
56% |
|
Diphenylphosphinic chloride; With hydroxylamine hydrochloride; sodium hydroxide; In 1,4-dioxane; for 0.583333h;
With sodium hydroxide; In water; for 0.5h;
|
47% |
|
With sodium hydroxide; hydroxylamine hydrochloride; In 1,4-dioxane; water; at 0 ℃; for 0.25h;
Diphenylphosphinic chloride; In 1,4-dioxane; water; at 0 ℃; for 0.25h;
With sodium hydroxide; In water; at 0 ℃; for 1h;
|
36% |
|
With sodium hydroxide; hydroxylamine hydrochloride; In 1,4-dioxane; water; for 0.0666667h; ice/salt bath;
|
5.8 g |
|
With hydroxylamine; In benzene; 1) 5 deg C, 0.3 h; 2) 5 deg C --> room temperature, 0.5 h;
|
|
|
With hydroxylamine hydrochloride; triethylamine; Yield given. Multistep reaction; 1) CH2Cl2, - 20 deg C, 1.5 h, 2) CH2Cl2, -20 deg C, 1.5 h;
|
|
|
With hydroxylamine hydrochloride; N-ethyl-N,N-diisopropylamine; In dichloromethane; for 1.05h; Cooling with methanol-ice;
Diphenylphosphinic chloride; In dichloromethane; at 0 ℃; for 2h;
|
|
|
With sodium hydroxide; hydroxylamine hydrochloride; In 1,4-dioxane; water; at -15 ℃; for 0.333333h;
|
|
|
With hydroxylamine hydrochloride; In 1,4-dioxane; sodium hydroxide; water;
|
|
|
With hydroxylamine hydrochloride; sodium hydrogencarbonate; In 1,4-dioxane; water; at 0 - 20 ℃; for 2.5h;
|
|
|
With hydroxylamine hydrochloride; sodium hydroxide; In 1,4-dioxane; water; at 0 - 10 ℃;
|
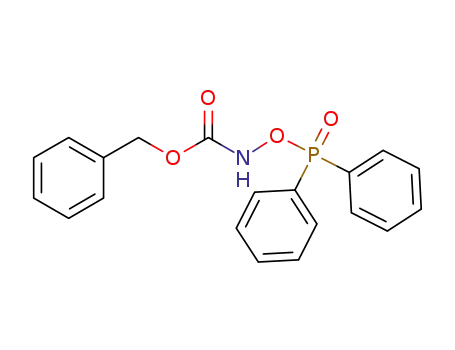
C20H18NO4P


O-(diphenylphosphinyl)hydroxylamine
| Conditions | Yield |
|---|---|
|
With palladium on activated charcoal; hydrogen; In tetrahydrofuran; at 35 ℃; for 4h;
|
92% |
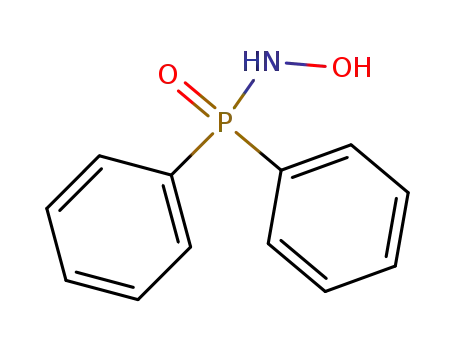
N-hydroxy-P,P-diphenylphosphinic amide
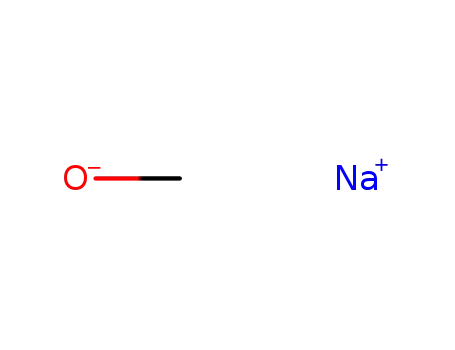
sodium methylate

Diphenylphosphinic chloride
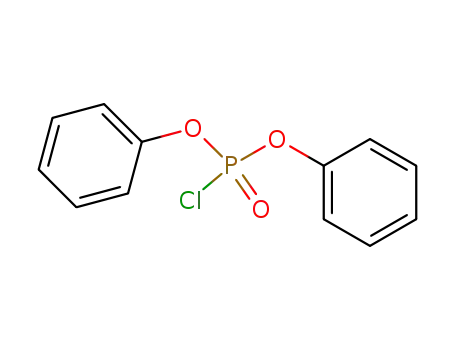
chlorophosphoric acid diphenyl ester
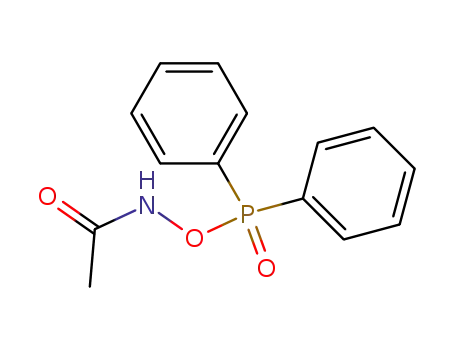
N-acetyl-O-(diphenylphosphinyl)hydroxylamine
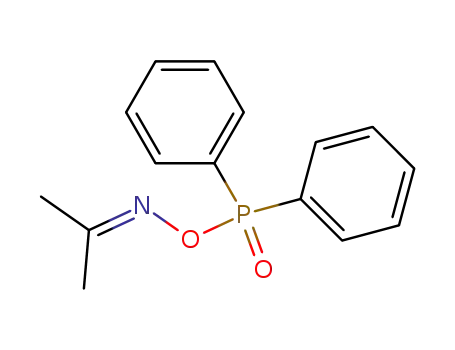
O-Dimethylformiminodiphenylphosphonate
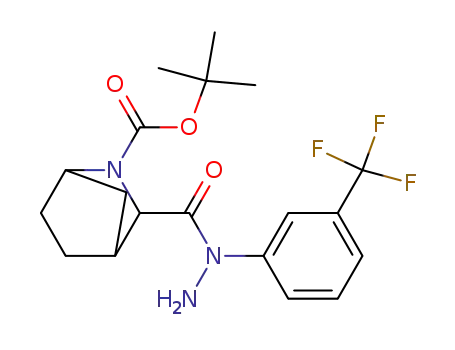
3-[[1-[3-(Trifluoromethyl)phenyl]hydrazino]carbonyl]-2-azabicyclo[2.2.1]heptane-2-carboxylic acid 1,1-dimethylethyl ester
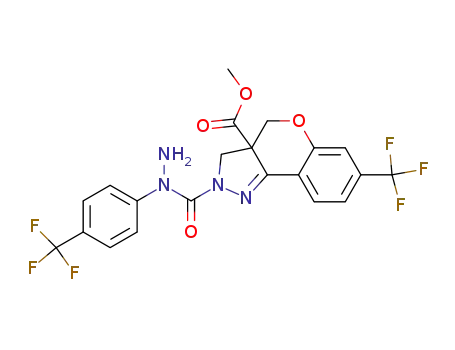
Methyl 2-[[-1-[4-(trifluoromethyl)phenyl]hydrazino]carbonyl]-2,3-dihydro-7-(trifluoromethyl)[1]benzopyrano[4,3-c]pyrazole-3a (4H)-carboxylate