Your Location:Home > Products > Pharmaceutical > Dapagliflozin

CasNo: 461432-26-8
Molecular Formula: C21H25ClO6
|
461432-26-8 Name |
|
|
Name |
Dapagliflozin |
|
Synonym |
DAPAGLIFLOZIN;(1S)-1,5-Anhydro-1-C-[4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl]-D-glucitol;Dapagliflozin propanediol;BMS-512148-05;Dapagliflozin S1548 Selleck;DAPAGLIFLOZIN BASE;Daglican;Dapagliflozi |
|
461432-26-8 Biological Activity |
|
|
Related Catalog |
Signaling Pathways >> Membrane Transporter/Ion Channel >> SGLT Research Areas >> Metabolic Disease |
|
Target |
SGLT2[1] |
|
461432-26-8 Chemical & Physical Properties |
|
|
Boiling point |
609.0±55.0 °C at 760 mmHg |
|
Density |
1.3±0.1 g/cm3 |
|
Molecular Formula |
C21H25ClO6 |
|
Molecular Weight |
408.873 |
|
Flash Point |
322.1±31.5 °C |
|
PSA |
99.38000 |
|
LogP |
4.42 |
|
Exact Mass |
408.133972 |
|
Vapour Pressure |
0.0±1.8 mmHg at 25°C |
|
Index of Refraction |
1.614 |
Dapagliflozin (Forxiga®) is a highly potent, reversible and selective sodium-glucose cotransporter-2 inhibitor indicated worldwide for the treatment of type 2 diabetes (T2D). Dapagliflozin therapy has been shown to impact a number of CV risk factors. Dapagliflozin improved glycemia with a low intrinsic propensity to cause hypoglycemia.In healthy subjects, dapagliflozin was rapidly absorbed after oral administration with a peak time Tmax being 1 to 2 hours, a protein binding rate of 91%, an oral bioavailability of about 78% and a plasma terminal half-life of 12.9 hours. Dapagliflozin was generally well tolerated, with a low risk of hypoglycaemia; diabetic ketoacidosis (DKA), although rare, and genital infections were more common with dapagliflozin than placebo.
Definition
ChEBI: A C-glycosyl comprising beta-D-glucose in which the anomeric hydroxy group is replaced by a 4-chloro-3-(4-ethoxybenzyl)phenyl group. Used (in the formo f its propanediol monohydrate) to improve glycemic ontrol, along with diet and exercise, in adults with type 2 diabetes.
InChI:InChI=1/C21H25ClO6/c1-2-27-15-6-3-12(4-7-15)9-14-10-13(5-8-16(14)22)21-20(26)19(25)18(24)17(11-23)28-21/h3-8,10,17-21,23-26H,2,9,11H2,1H3/t17-,18-,19+,20-,21+/m1/s1
In a prespecified analysis of the DELIVER (Dapagliflozin Evaluation to Improve the Lives of Patients With Preserved Ejection Fraction Heart Failure) trial, we examine effects of dapagliflozin on health status using the Kansas City Cardiomyopathy Questionnaire (KCCQ). The clinical benefits of dapagliflozin in HFmrEF/HFpEF appear especially pronounced in those with greater baseline symptom impairment.
Access to unprotected (hetero)aryl pseudo-C-glucosides via a mild Pd-catalysed Hiyama cross-coupling reaction of protecting-group-free 1-diisopropylsilyl-d-glucal with various (hetero)aryl halides has been developed. Finally, the versatility of our methodology was proved by the synthesis of other analogues of dapagliflozin.
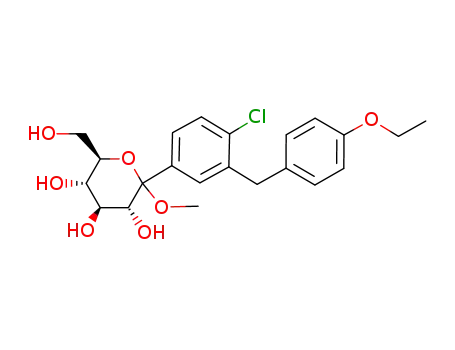
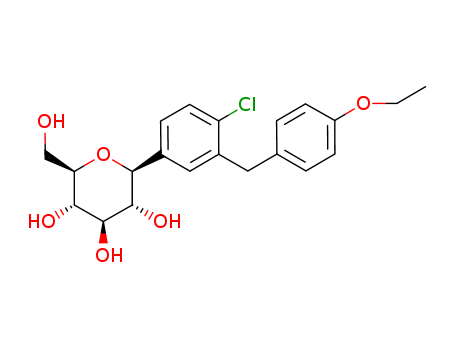
(3R,4S,5S,6R)-2-(4-chloro-3-(4-ethoxybenzyl)phenyl)-6-(hydroxymethyl)-2-methoxytetrahydro-2H-pyran-3,4,5-triol

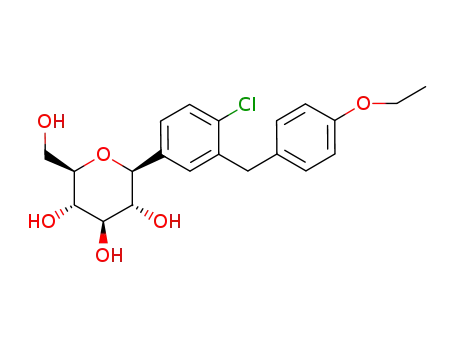
dapagliflozin
| Conditions | Yield |
|---|---|
|
With triethylsilane; boron trifluoride diethyl etherate; In methanol; dichloromethane; ethyl acetate; at -20 - 20 ℃; for 5h;
|
83.1% |
|
(3R,4S,5S,6R)-2-(4-chloro-3-(4-ethoxybenzyl)phenyl)-6-(hydroxymethyl)-2-methoxytetrahydro-2H-pyran-3,4,5-triol; With triethylsilane; boron trifluoride diethyl etherate; In dichloromethane; acetonitrile; at -10 - 20 ℃;
With water; sodium hydrogencarbonate; In dichloromethane; acetonitrile;
|
44% |
|
With triethylsilane; boron trifluoride diethyl etherate; In dichloromethane; acetonitrile; at -10 ℃; for 1.5h; Temperature; Time; Inert atmosphere;
|
|
|
Multi-step reaction with 3 steps
1: boron trifluoride diethyl etherate; triethylsilane / dichloromethane; acetonitrile / -45 - -40 °C
2: N-ethyl-N,N-diisopropylamine; dmap / dichloromethane / 0 - 5 °C
3: sodium hydroxide; water / tetrahydrofuran; methanol / 24 h
With triethylsilane; dmap; boron trifluoride diethyl etherate; water; N-ethyl-N,N-diisopropylamine; sodium hydroxide; In tetrahydrofuran; methanol; dichloromethane; acetonitrile;
|
|
|
With triethylsilane; boron trifluoride diethyl etherate; In dichloromethane; at -40 - -5 ℃; for 6h; Temperature;
|
|
|
Multi-step reaction with 3 steps
1: triethylsilane; boron trifluoride diethyl etherate / dichloromethane / 1.5 h / -10 - 0 °C / Inert atmosphere
2: water / 45 - 50 °C / Inert atmosphere
3: tert-butyl methyl ether; n-heptane / -15 - 50 °C / Inert atmosphere
With triethylsilane; boron trifluoride diethyl etherate; In n-heptane; dichloromethane; tert-butyl methyl ether; water;
|
|
|
With triethylsilane; boron trifluoride diethyl etherate; In dichloromethane; at -30 - 20 ℃; for 1h;
|
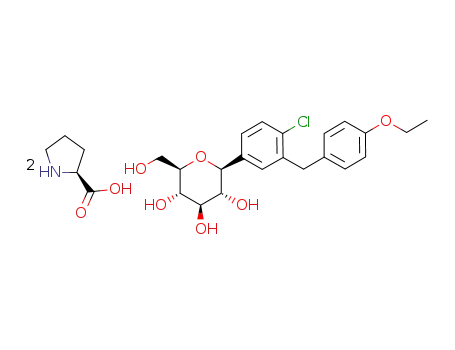
(2S,3R,4R,5S,6R)-2-(4-chloro-3-(4-ethoxybenzyl)phenyl)-6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol bis(L-proline) cocrystal


dapagliflozin
| Conditions | Yield |
|---|---|
|
In water; ethyl acetate; at 80 ℃;
|
95% |
|
With water; In methanol; at 20 - 25 ℃; for 16h;
|
94% |
|
With water; In methanol; at 20 - 25 ℃; for 17h; Reflux;
|
94% |
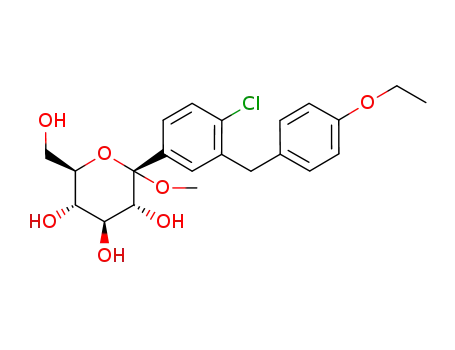
(2S,3R,4S,5S,6R)-2-[4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl]-6-(hydroxymethyl)-2-methoxy-tetrahydropyran-3,4,5-triol
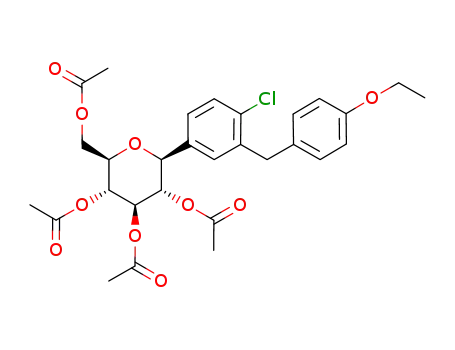
(2R,3R,4R,5S,6S)-2-(acetoxymethyl)-6-(4-chloro-3-(4-ethoxybenzyl)phenyl)tetrahydro-2H-pyran-3,4,5-triyl triacetate

(3R,4S,5S,6R)-2-(4-chloro-3-(4-ethoxybenzyl)phenyl)-6-(hydroxymethyl)-2-methoxytetrahydro-2H-pyran-3,4,5-triol
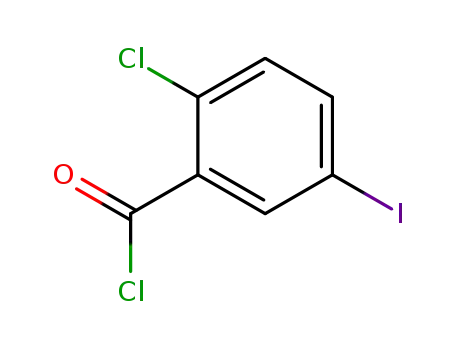
2-chloro-5-iodobenzoylchloride
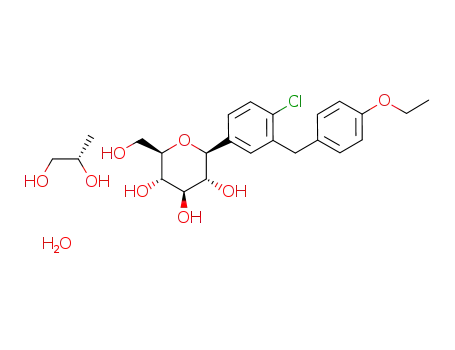
dapagliflozin (S) propylene glycol hydrate
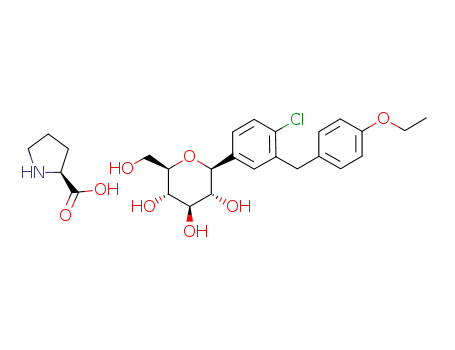
L-proline (2S,3R,4R,5S,6R)-2-[4-chloro-3-(4-ethoxybenzyl)phenyI]-6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol complex
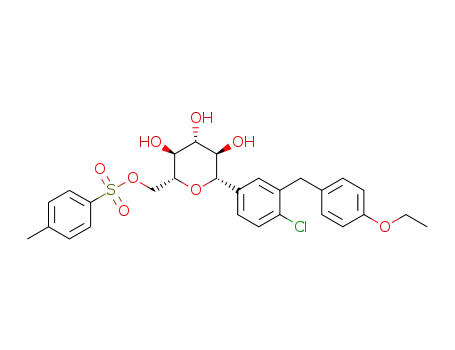
((2R,3S,4R,5R,6S)-6-(4-chloro-3-(4-ethoxybenzyl)phenyl)-3,4,5-trihydroxytetrahydro-2H-pyran-2-yl)methyl 4-methylbenzenesulfonate
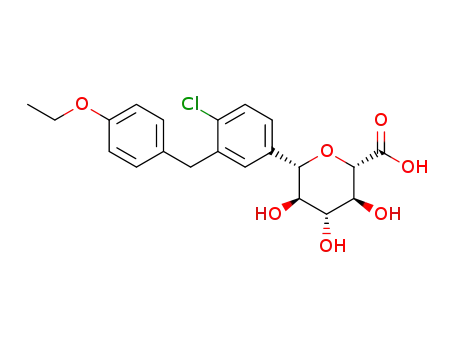
(2S,3S,4R,5R,6S)-6-[4-chloro-3-(4-ethoxy-benzyl)-phenyl]-3,4,5-trihydroxy-tetrahydro-pyran-2-carboxylic acid