Your Location:Home > Products > Acetic acid, 2-oxo-,ethyl ester
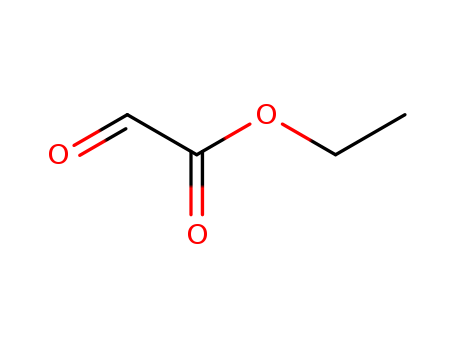

CasNo: 924-44-7
Molecular Formula: C4H6O3
Appearance: Clear colorless to slightly yellow solution
Clear colorless to slightly yellow solution. Ethyl glyoxylate is widely used as an intermediate in the synthesis of pharmaceuticals (high reactivity of aldehyde function). It is used in the synthesis of a biodegradable polymer, poly(ethyl glyoxylate). The Ru(II)-catalyzed regioselective[4+1] cycloaddition of azobenzenes with ethyl glyoxalate through C-H bond activation has been developed. It is actively involved in the Friedel-Crafts alkylation reactions with thiophenes to give the corresponding secondary alcohols. Different activated aldehydes like ethyl glyoxalate and 2,2,2-trifluoroacetaldehyde also efficiently underwent nucleophilic addition with 2-arylindazoles.
The three-component reaction of ethyl glyoxalate, 2-aminopyridines, and cyclic 1,3-dicarbonyl compounds such as 4-hydroxycoumarin, N,N-dimethylbarbituric acid and 4-hydroxy-6-methyl-2H-pyran-2-one is described for the synthesis of 3-hydroxyimidazo[1,2-a]pyridine zwitterion derivatives.
Definition
ChEBI: The ethyl ester of glyoxylic acid.
Synthesis Reference(s)
Tetrahedron Letters, 24, p. 2223, 1983 DOI: 10.1016/S0040-4039(00)81889-4
InChI:InChI=1/C4H6O3/c1-2-7-4(6)3-5/h3H,2H2,1H3
β-Nitro acrylic esters, obtained by the reaction of nitroalkanes and ethyl glyoxalate, are the key building blocks for the immediate synthesis of both the title compounds. In fact, their treatment with titanium trichloride produce the direct conversion to the β-keto esters, while their reaction with sodium boron hydride gives the one-pot synthesis of α,β-unsaturated esters through formal substitution of the vinylic nitro group with an hydrogen.
The CuOBA material was used as an efficient heterogeneous catalyst for the coupling reaction of phenyl acetylene and ethyl glyoxalate in the presence of 1 equivalent morpholine as a base to form 1.2-dicarbonyl-3-ene product. The yield of transformation was more than 90% at 80oC with 9 mol% CuOBA catalyst after 4 hours. The results showed that CuOBA could be recovered and reused seven times without a significant degradation in its catalytic activity.
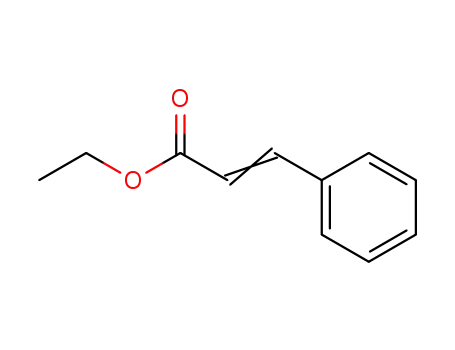
ethyl 3-phenyl-2-propenoate

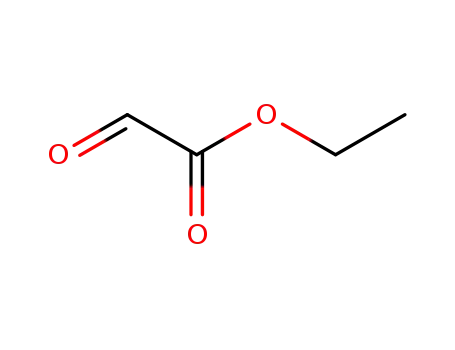
glyoxylic acid ethyl ester

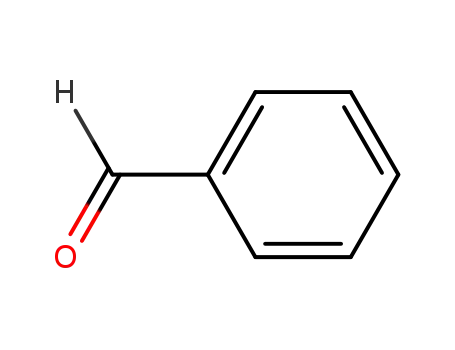
benzaldehyde
| Conditions | Yield |
|---|---|
|
With phosphoric acid; oxygen; 2-methyl-5-tert-butylthiophenol; methyl 2-N-salicylidene-3-hydroxypropanoate; manganese(ll) chloride; In acetone; at 20 ℃; for 3h; under 760 Torr; Title compound not separated from byproducts;
|
53 % Chromat. |
tetrachloromethane

tris-(2-chloro-ethyl)-amine


glyoxylic acid ethyl ester

Glyoxal

N,N-bis(chloro-2-ethyl)amine
| Conditions | Yield |
|---|---|
|
|
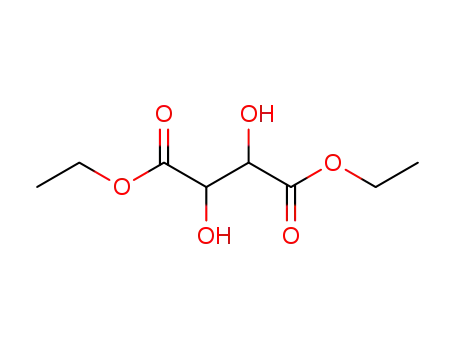
Diethyl tartrate
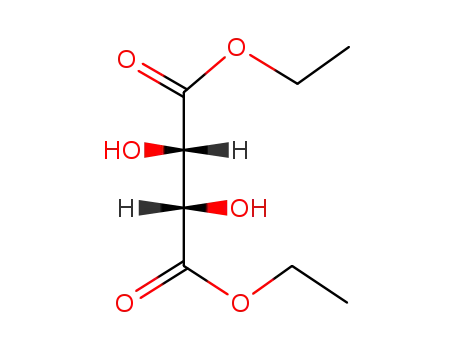
(+/-)-diethyl tartrate
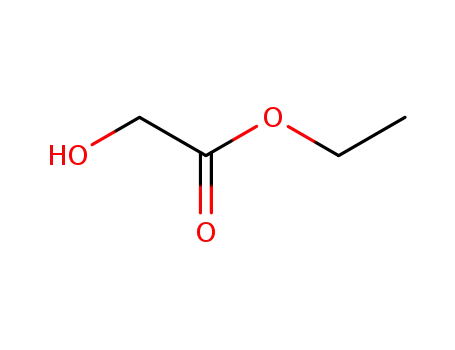
ethyl 2-hydroxyacetate
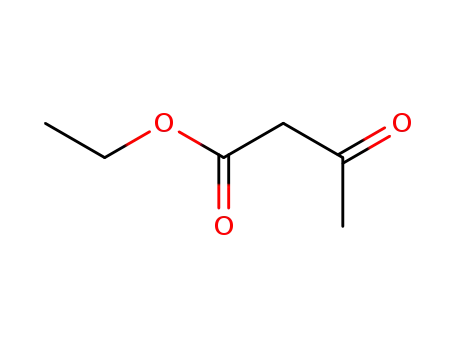
ethyl acetoacetate
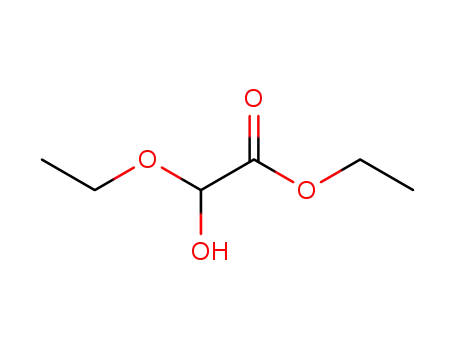
ethyl 2-ethoxyglycolate
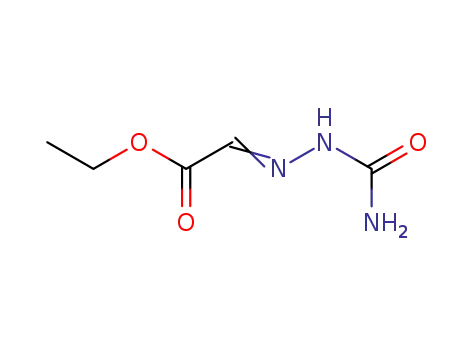
ethyl 2-(2-carbamoylhydrazono)acetate
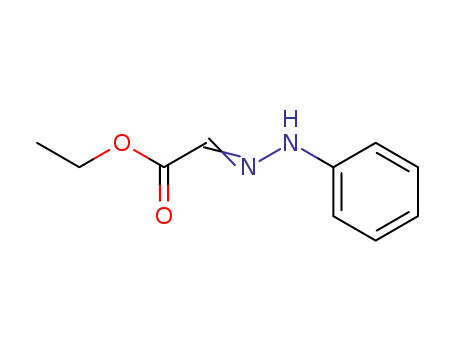
phenylhydrazone of ethyl ester of glyoxalic acid
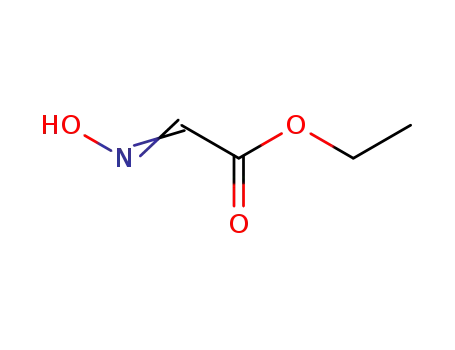
ethyl 2-(hydroxyimino)acetate