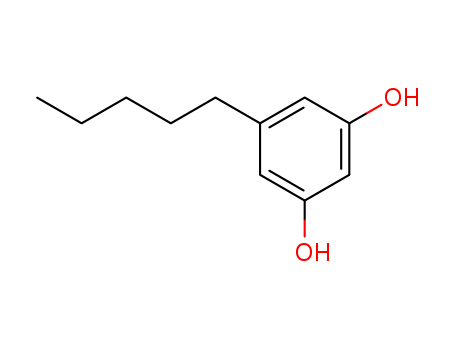

CasNo: 500-66-3
Molecular Formula: C11H16O2
Appearance: light purple to brown crystalline mass
Olivetol (OLV), known as 3, 5-dihydroxypentylene, was a natural polyphenol compound that could be found in lichens, some insects, and many plants, is an important polyphenol compound and intermediate in the synthesis of cannabinoids possessing many types of biological activities. It is used in various methods to produce synthetic analogues of cannabinoids. Olivetol is one of the first intermediate involved in the cannabinoid biosynthesis in Cannabis sativa. OLV structure is found in cannabinoids, so OLV could be used as an analogue of CBD as it could mimic its interactions with the drug delivery systems.
InChI:InChI=1/C11H16O2/c1-2-3-4-5-9-6-10(12)8-11(13)7-9/h6-8,12-13H,2-5H2,1H3
To the best of our knowledge, there was a rare report of the electrochemical sensor based on CPE for the determination of olivetol. In this study, CuO nanoparticles and L-serine were modified on CPE, resulting in the p-L-serine/CuO/CPE for the sensitive detection of olivetol in the electrochemical method.
Olivetolic acid is thus a central intermediate in the biosynthesis of the phytocannabinoids, while olivetolic acid and more commonly olivetol are crucial building blocks in the chemical synthesis of phytocannabinoids and analogues. In conclusion, the synthesis of olivetolic acid methyl ester 3 has been achieved in 65% isolated yield in only two steps from the cyclic diketone 8 employing an oxidative aromatization strategy that is catalytic in iodine in DMSO.
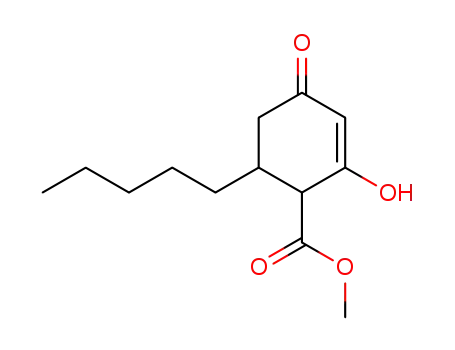
methyl 6-n-pentyl-2-hydroxy-4-oxo-cyclohex-2-ene-1-carboxylate

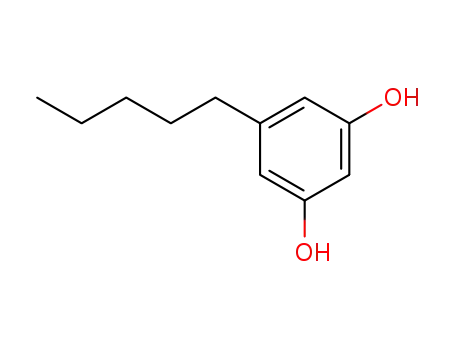
Olivetol
| Conditions | Yield |
|---|---|
|
With bromine; In N,N-dimethyl-formamide; at 160 ℃; for 10h; Cooling with ice;
|
69.3% |
|
With bromine; In N,N-dimethyl-formamide; at 80 - 160 ℃;
|
69.3% |
|
With bromine; In N,N-dimethyl-formamide; at 160 ℃; for 11.5h; Cooling with ice;
|
69.3% |
|
With bromine; In N,N-dimethyl-formamide; at 160 ℃; for 10h; Cooling with ice;
|
69.3% |
|
With bromine; In N,N-dimethyl-formamide; at 80 - 160 ℃; for 11.5h; Cooling with ice;
|
69.3% |
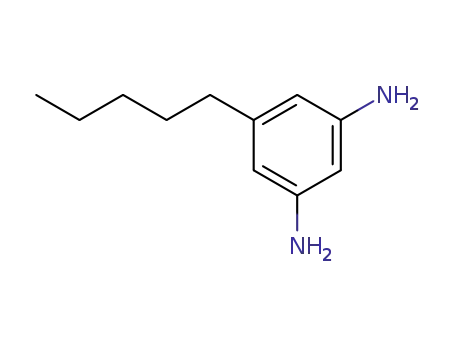
3,5-diamino-1-pentylbenzene


Olivetol
| Conditions | Yield |
|---|---|
|
3,5-diamino-1-pentylbenzene; With sulfuric acid; sodium nitrite; In dichloromethane; at 5 ℃;
With sulfuric acid; sodium sulfate; In water; for 1h; Reflux;
|
78% |
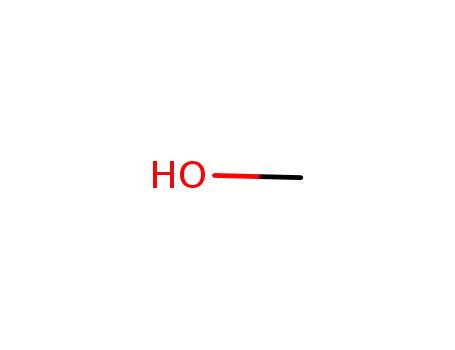
methanol
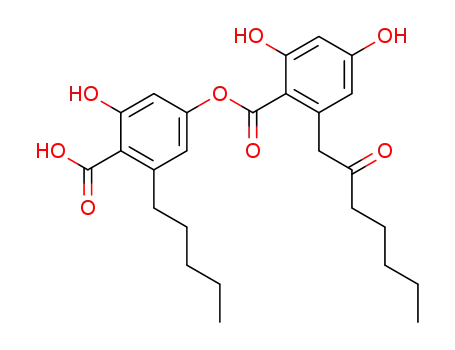
olivetoric acid
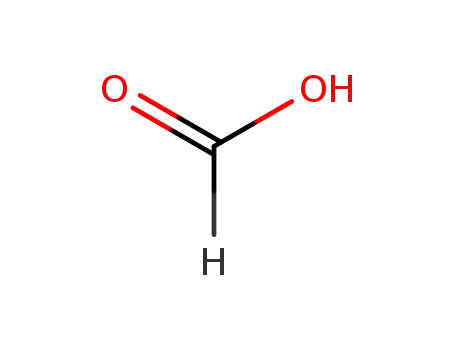
formic acid
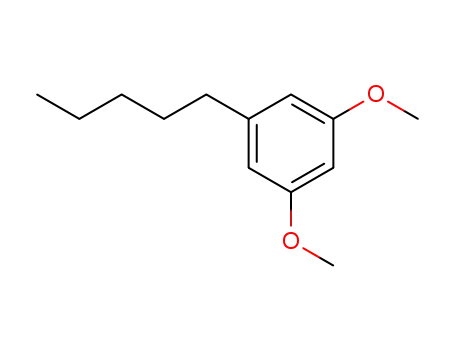
1,3-dimethoxy-5-pentylbenzene
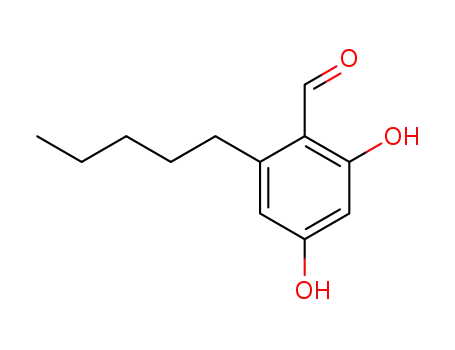
2,4-dihydroxyl-6-pentylbenzaldehyde
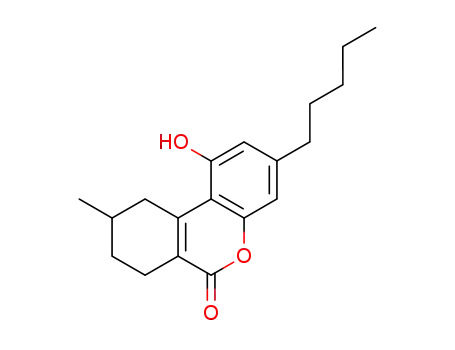
(+/-)-1-hydroxy-9-methyl-3-pentyl-7,8,9,10-tetrahydro-benzo[c]chromen-6-one
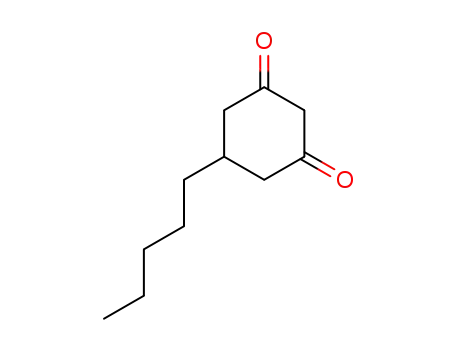
5-Pentyl-1,3-cyclohexanedione
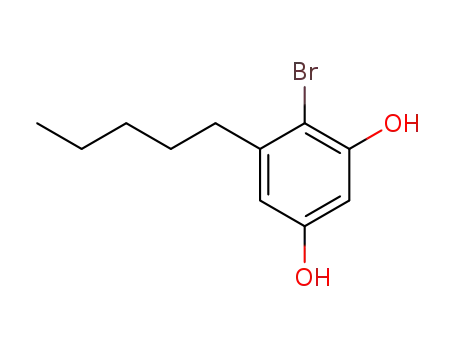
4-bromo-5-pentylbenzene-1,3-diol