Your Location:Home > Products > 2-Bromo-4-chloro-1-iodobenzene
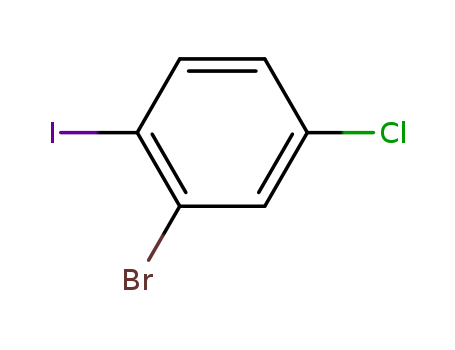

CasNo: 31928-44-6
Molecular Formula: C6H3BrClI
|
31928-44-6 Name |
|
|
Name |
2-Bromo-4-chloro-1-iodobenzene |
|
Synonym |
2-BROMO-4-CHLORO-1-IODOBENZENE;2-Bromo-1-iodo-4-chlorobenzene;1-Iodo-2-bromo-4-chlorobenzene;2-Bromo-4-chloro-1-iodobenzene >;Benzene, 2-bromo-4-chloro-1-iodo- |
|
31928-44-6 Chemical & Physical Properties |
|
|
Melting point |
32-35°C |
|
Boiling point |
278.9±20.0 °C at 760 mmHg |
|
Density |
2.3±0.1 g/cm3 |
|
Molecular Formula |
C6H3BrClI |
|
Molecular Weight |
317.349 |
|
Flash Point |
122.5±21.8 °C |
|
LogP |
4.41 |
|
Exact Mass |
315.815125 |
|
Vapour Pressure |
0.0±0.6 mmHg at 25°C |
|
Index of Refraction |
1.663 |
2-Bromo-4-chloro-1-iodobenzene is a compound of Organic Building Blocks research chemicals for sale.
InChI:InChI=1/C6H3BrClI/c7-5-3-4(8)1-2-6(5)9/h1-3H
It is noteworthy that the reactions of 2-bromo-4-chloro-1-iodobenzene and 4-chloro-1,2-diiodobenzene resulted in a mixture of two regioisomers in both cases in a high total yield (Table …An environmentally benign and efficient method has been developed for the synthesis of phenothiazines via a tandem iron-catalyzed C–S/C-N cross-coupling reaction.
A one-pot universal approach for transforming arylamines to aryl halides via reaction with sodium nitrite (NaNO2) and N-halosuccinimide (NXS) in DMF at room temperature under metal- and acid-free condition is described. This new protocol that is complementary to the Sandmeyer reaction, is suggested to involve the in situ generation of nitryl halide induce nitrosylation of aryl amine to form the diazo intermediate which is halogenated to furnish the aryl halide.
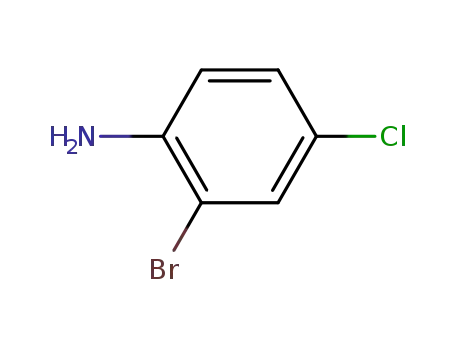
4-chloro-2-bromoaniline

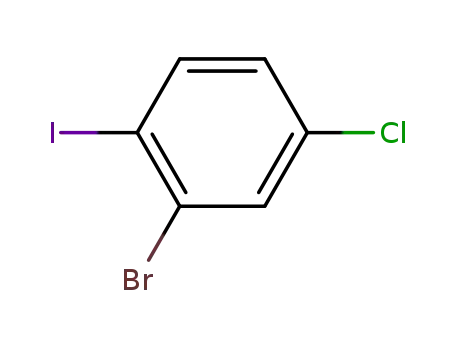
2-bromo-4-chloroiodobenzene
| Conditions | Yield |
|---|---|
|
4-chloro-2-bromoaniline; With hydrogenchloride; sodium nitrite; In water; at -15 - 0 ℃; for 0.833333h;
With potassium iodide; In water; at 20 ℃; for 6.17h;
|
81% |
|
With N-iodo-succinimide; sodium nitrite; In N,N-dimethyl-formamide; at 20 ℃; for 4h;
|
78% |
|
4-chloro-2-bromoaniline; With hydrogenchloride; In water; at 80 ℃; for 1h;
With sodium nitrite; In water; at -5 ℃; for 1.5h;
With potassium iodide; In water; at 0 - 20 ℃; for 40h;
|
67% |
|
4-chloro-2-bromoaniline; With hydrogenchloride; sodium nitrite; at 0 ℃; for 0.5h;
With potassium iodide; In water; at 20 ℃; for 12h; Further stages.;
|
50% |
|
4-chloro-2-bromoaniline; With hydrogenchloride; In water; at 80 ℃;
With sodium nitrite; In water; at 0 ℃; for 1h;
With potassium iodide; In water; at 10 - 60 ℃;
|
50% |
|
With sulfuric acid; potassium iodide; sodium nitrite; Multistep reaction; 1.) water, 10 deg C, 30 min, 2.) water 60 deg C;
|
|
|
4-chloro-2-bromoaniline; With sulfuric acid; sodium nitrite; In water; at 0 ℃; for 2h; Inert atmosphere;
With copper(l) iodide; potassium iodide; In water; at 0 - 20 ℃; Inert atmosphere;
|
18.8 g |
|
With hydrogenchloride; potassium iodide; sodium nitrite; In water; at -15 - 20 ℃; for 18.5h;
|
16.4 g |
|
Multi-step reaction with 2 steps
1: tert.-butylnitrite / dichloromethane; tetrahydrofuran / 0.58 h / -20 - 0 °C
2: pyridine; iodine / acetonitrile / 2 h / -30 - 25 °C
With pyridine; tert.-butylnitrite; iodine; In tetrahydrofuran; dichloromethane; acetonitrile;
|
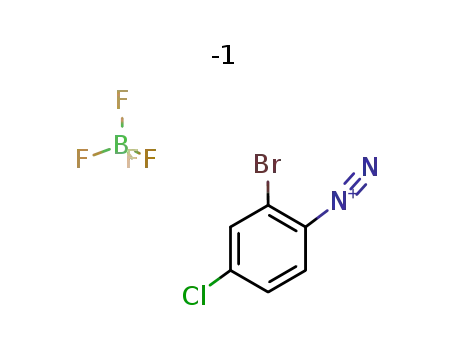
2-bromo-4-chlorobenzenediazonium tetrafluoroborate


2-bromo-4-chloroiodobenzene
| Conditions | Yield |
|---|---|
|
With pyridine; iodine; In acetonitrile; at -30 - 25 ℃; for 2h;
|
63% |

4-chloro-2-bromoaniline
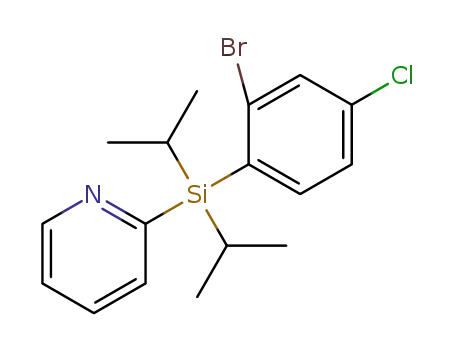
C17H21BrClNSi
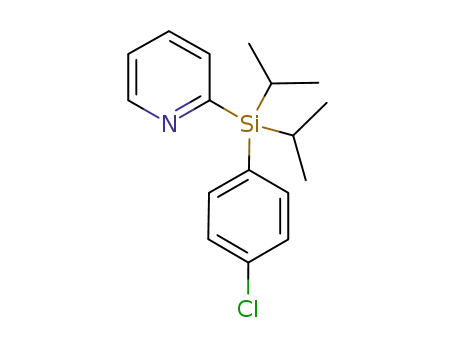
2-((4-chlorophenyl)diisopropylsilyl)pyridine
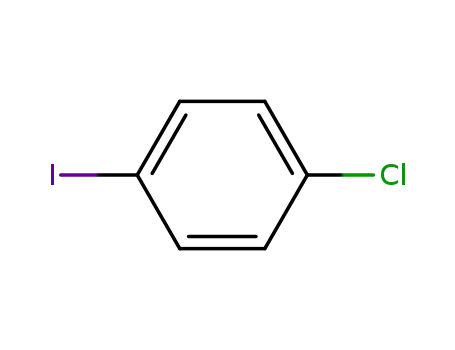
1-Chloro-4-iodobenzene
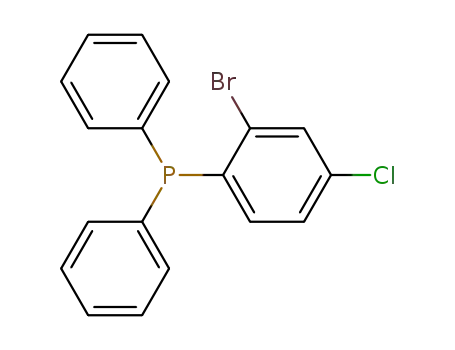
(2-bromo-4-chlorophenyl)diphenylphosphine
2-chloro-N,N-dimethyl-10H-phenothiazine-10-propanamine

C44H25N

C42H25N