Your Location:Home > Products > Fine Chemicals > 1-Adamantanamine hydrochloride
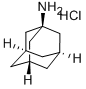

CasNo: 665-66-7
Molecular Formula: C10H18ClN
Appearance: Crystalline solid
|
665-66-7 Name |
|
|
Name |
1-Adamantanamine hydrochloride |
|
Synonym |
Amantadine hydrochloride,1-Adamantanamine hydrochloride, 1-Adamantylamine hydrochloride, 1-Aminoadamantane hydrochloride;Amantadine Hydrochloride (200 mg);1-AdaMantanaMine hydrochloride, 99+% 100GR;1-AdaMantanaMine hydrochloride, 99+% 25GR;1-AdaMantanaMine hydrochloride, 99+% 5GR;1-adamantane amine hydrochloride;1-Adamantanamine Hydrochleride;1-Aminoadamantane Hydrochloride Amantadine Hydrochloride |
|
665-66-7 Biological Activity |
|
|
Related Catalog |
Signaling Pathways >> Anti-infection >> Influenza Virus Research Areas >> Infection |
|
665-66-7 Chemical & Physical Properties |
|
|
Melting point |
>300 °C(lit.) |
|
Boiling point |
225.7ºC at 760 mmHg |
|
Density |
1.067g/cm3 |
|
Molecular Formula |
C10H18ClN |
|
Molecular Weight |
187.710 |
|
Flash Point |
96ºC |
|
PSA |
26.02000 |
|
LogP |
3.41620 |
|
Exact Mass |
187.112778 |
|
Index of Refraction |
1.558 |
|
Water Solubility |
soluble |
1-Adamantanamine hydrochloride 665-66-7, antiviral, antiparkinsonian; treatment of drug-induced extrapyrimidal reactions, is Crystalline Solid used in the prophylactic or symptomatic treatment of influenza A. It is also used as an antiparkinsonian agent, to treat extra pyramidal reactions, and for postherpetic neuralgia. 1-adamantanamine hydrochloride (ATH) is introduced to the upper surface of the perovskite film to heal the defects of the perovskite surface.
InChI:InChI=1/C10H17N/c11-10-4-7-1-8(5-10)3-9(2-7)6-10/h7-9H,1-6,11H2/t7-,8+,9-,10-
With obvious improvement, VOC and FF of 1.159 V and 0.796 for the control device are raised to 1.178 V and 0.826 for the ATH-modified device, respectively. Finally, during an operational stability measurement of more than 1000 h, the ATH-treated PSC exhibited better moisture resistance, thermal persistence, and light stability.
Compared, the prior art . the synthesis method: the amantadine hydrochloride has an important clinical value and market value Ritter, and the synthesis process, of the hydrochloric acid adamantanamine, effectively improves the industrial application potential, of the hydrochloric acid adamantane hydrochloride synthesis process as, by adopting a special process and a component. The method mainly, comprises, steps of preparation of an amantadine hydrochloride synthesis process by using a special process and a method. (by machine translation)
adamantane

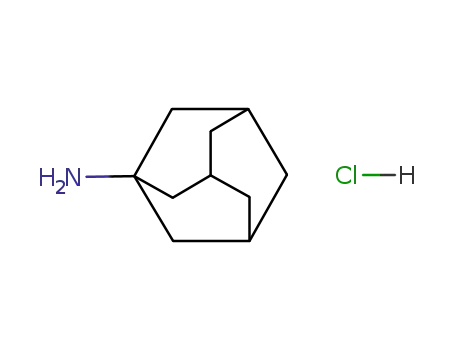
amantadine hydrochloride

adamantan-2-amine hydrochloride
| Conditions | Yield |
|---|---|
|
adamantane; With methanol; cerium(III) chloride; di-tert-butyl-diazodicarboxylate; tetrabutyl-ammonium chloride; In acetonitrile; at 20 ℃; for 10h; Irradiation; Inert atmosphere; Sealed tube;
With trifluoroacetic acid; In dichloromethane; at 20 ℃; for 1h; Reagent/catalyst; Further stages;
|
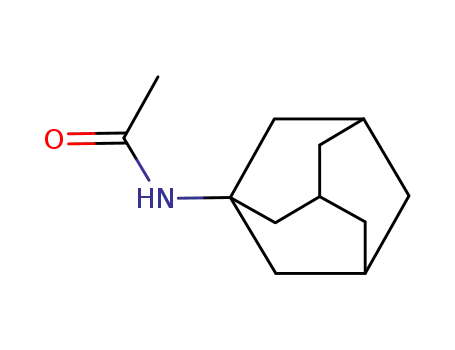
N-(1-adamantyl)acetamide

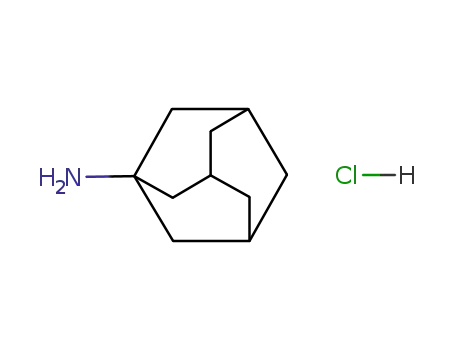
amantadine hydrochloride
| Conditions | Yield |
|---|---|
|
N-(1-adamantyl)acetamide; With sodium heptadecanoic acid; fipronilβ-cyclodextrin; sodium hydroxide; In ethanol; water; for 20h; Reflux;
With hydrogenchloride; In water; at 70 ℃;
|
96.5% |
|
N-(1-adamantyl)acetamide; With propylene glycol; potassium hydroxide; In water; at 125 - 130 ℃; for 8h; Green chemistry;
With hydrogenchloride; In dichloromethane; water; at 55 - 60 ℃; for 1h; Temperature; Time; Green chemistry;
|
82% |
|
N-(1-adamantyl)acetamide; With methanol; water; sodium hydroxide; at 145 ℃; for 8h; Autoclave;
With hydrogenchloride; In dichloromethane; water; at 50 ℃; for 0.5h; Autoclave;
|
82.2% |
|
N-(1-adamantyl)acetamide; With pyridine; oxalyl dichloride; In tetrahydrofuran; at 0 ℃; for 0.5h; Inert atmosphere;
With propylene glycol; In tetrahydrofuran; at 0 - 25 ℃; Inert atmosphere;
With hydrogenchloride; In ethanol;
|
|
|
N-(1-adamantyl)acetamide; With tetrabutylammomium bromide; sodium hydroxide; In water; ethylene glycol; at 190 ℃; for 12h; Autoclave;
With hydrogenchloride; In water; ethylene glycol; pH=4;
|
|
|
N-(1-adamantyl)acetamide; With tetrabutylammomium bromide; sodium hydroxide; In water; at 190 ℃; for 12h; Autoclave;
With hydrogenchloride; In water; pH=4;
|
|
|
Multi-step reaction with 2 steps
1: sodium hydroxide / 1,2-dimethoxyethane / 12 h / 135 °C
2: hydrogenchloride / dichloromethane; water / 3 h / 50 °C
With hydrogenchloride; sodium hydroxide; In 1,2-dimethoxyethane; dichloromethane; water;
|
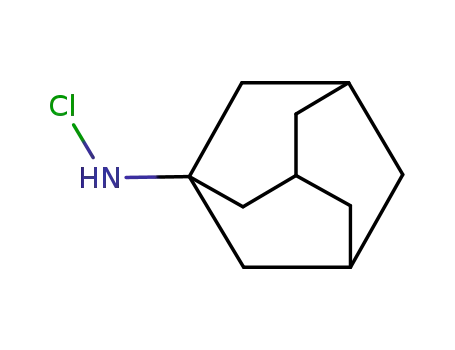
1-(Chloroamino)adamantane
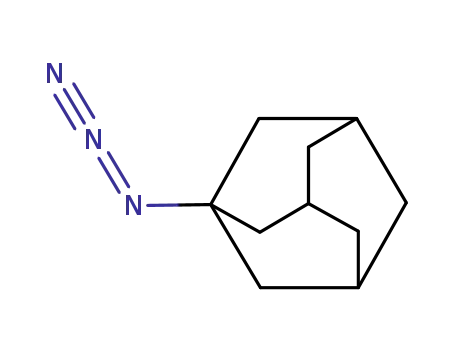
1-Azidoadamantane
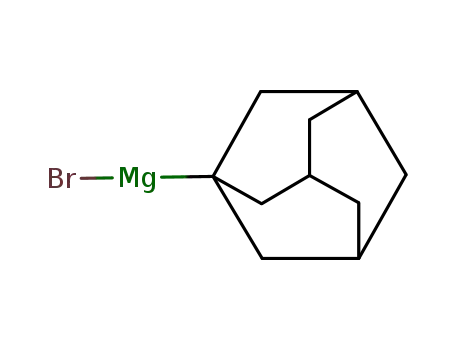
adamantylmagnesium bromide
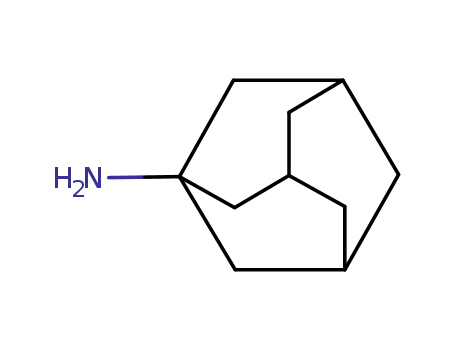
1-Adamantanamine
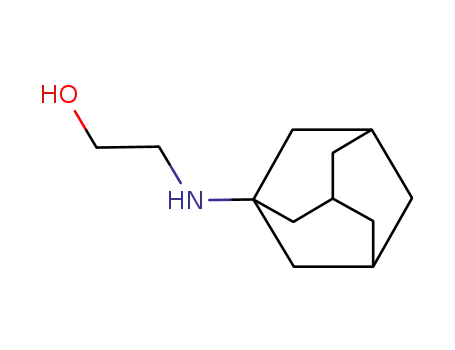
1-(2-hydroxyethylamino)adamantane
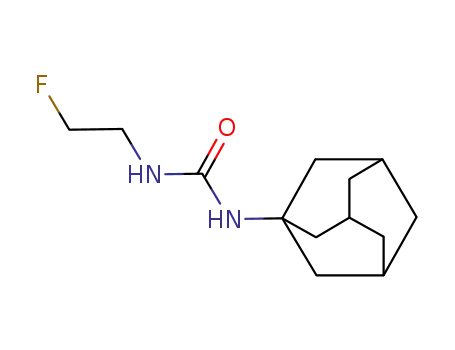
N-Adamantan-1-yl-N'-<2-fluor-aethyl>-harnstoff

N-(-adamantan-1-yl)-1-phenylmethanimine
N-(1-adamantyl)-2-chloroacetamide