Your Location:Home > Products > Cannabis > 5-Propyl-1,3-benzenediol
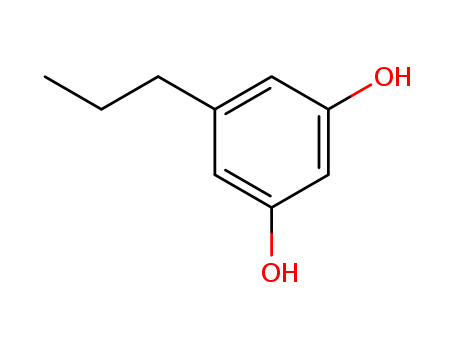

CasNo: 500-49-2
Molecular Formula: C9H12O2
|
500-49-2 Name |
|
|
Name |
5-Propyl-1,3-benzenediol |
|
Synonym |
5-Propyl-1,3-benzenediol;Divarinol;1,3-Benzenediol, 5-propyl-;3,5-Dihydroxypropylbenzene;1,3-Benzenediol, 5-propyl- CAS NO.500-49-2;3,5-Dihydroxy-1-propylbenzene |
|
500-49-2 Chemical & Physical Properties |
|
|
Melting point |
82.8°C |
|
Boiling point |
284.8ºC at 760 mmHg |
|
Density |
1.122g/cm3 |
|
Molecular Formula |
C9H12O2 |
|
Molecular Weight |
152.19000 |
|
Flash Point |
137.9ºC |
|
PSA |
40.46000 |
|
LogP |
2.05030 |
|
Exact Mass |
152.08400 |
|
Index of Refraction |
1.565 |
5-Propyl-1,3-benzenediol is a chemical compound that is found in cannabis. It has been shown to have anti-inflammatory and neuroprotective effects in vitro. It is an intermediate used to prepare Cannabigerovarin (C175140) which stimulates thermosensitive transient receptor potential (TRP) channels of vanilloid type-4 (TRPV4)-mediated [Ca2+]i with moderate-high efficacy (30-60% of the effect of ionomycin) and potency (EC50 0.9-6.4 μM),
InChI:InChI=1/C9H12O2/c1-2-3-7-4-8(10)6-9(11)5-7/h4-6,10-11H,2-3H2,1H3
The precursors 5‑butylbenzene‑1,3‑diol and 5‑propylbenzene‑1,3‑diol were synthesized as reported by our group for the 5‑hexylbenzene‑1,3‑diol synthesis (Ref. CBDH) (Scheme 1). Commercially available “pure” CBG was analyzed in the present work by HPLC coupled to UV and HRMS, highlighting the presence of two main impurities, which were identified as cannabigerovarin (CBGV) and cannabigerobutol (CBGB), the propyl and butyl homologs of CBG, respectively.
Cannabidiol is one of the main non-psychoactive cannabinoids present in Cannabis sativa and, in the last decade, it is gaining great interest among the scientific community for its pharmaceutical, nutraceutical, and cosmetic applications. This approach permits to synthesize products in very good yields (55–59 %), limiting the formation of psychoactive and illegal cannabinoids such as tetrahydrocannabinol (THC).
A revised modular approach to various synthetic (–)-trans-Δ8-THC derivatives through late-stage Suzuki–Miyaura cross-coupling reactions is disclosed. Importantly, we demonstrate that a para-bromo-substituted THC scaffold for Suzuki–Miyaura cross-coupling reactions has been initially reported incorrectly in recent literature.
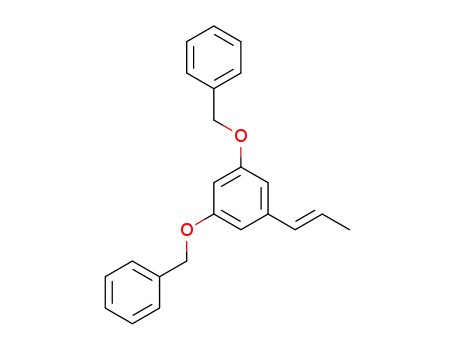
(E)-(((5-(prop-1-en-1-yl)-1,3-phenylene)bis(oxy))bis(methylene))dibenzene

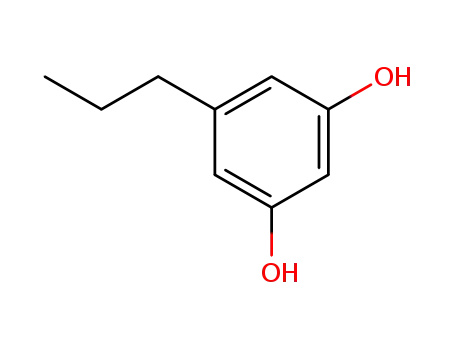
5-propyl-1,3-benzenediol
| Conditions | Yield |
|---|---|
|
With palladium 10% on activated carbon; hydrogen; In methanol; ethyl acetate;
|
96.3% |
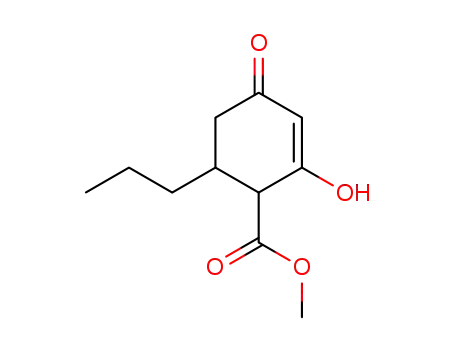
methyl 6-n-propyl-2-hydroxy-4-oxo-cyclohex-2-ene-1-carboxylate


5-propyl-1,3-benzenediol
| Conditions | Yield |
|---|---|
|
With bromine; In N,N-dimethyl-formamide; at 80 - 160 ℃; Cooling with ice;
|
|
|
With bromine; In N,N-dimethyl-formamide; at 160 ℃; for 10h; Cooling with ice;
|
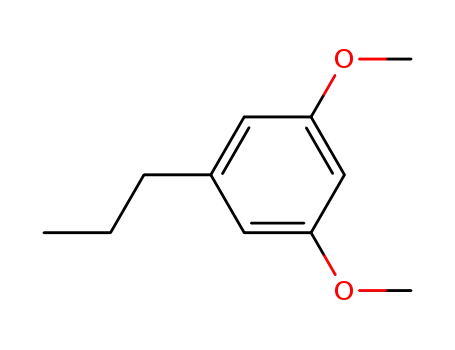
1,3-dimethoxy-5-propylbenzene
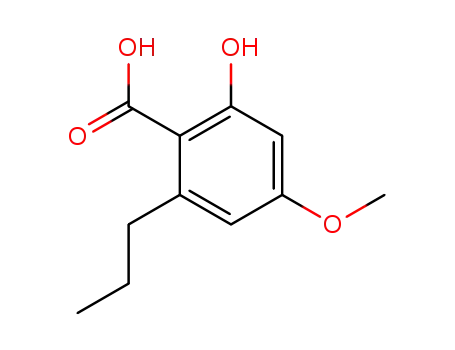
2-hydroxy-4-methoxy-6-propylbenzoic acid
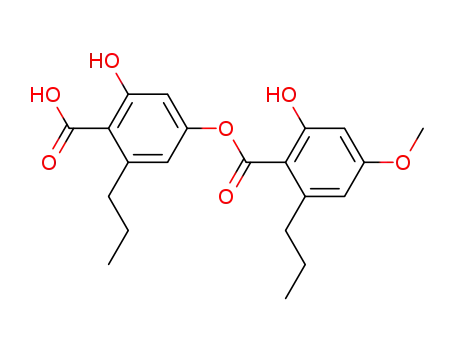
divaricatic acid
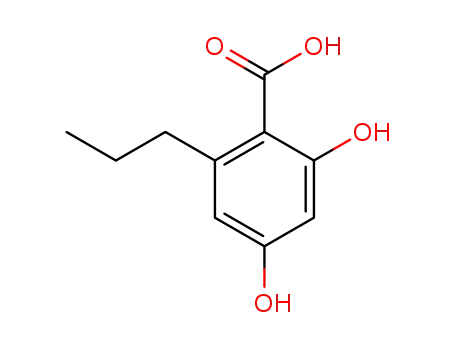
2,4-dihydroxy-6-propylbenzoic acid
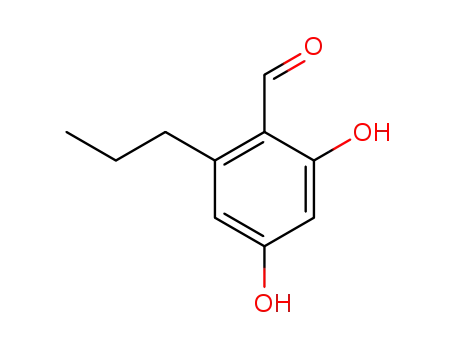
2,4-dihydroxy-6-propylbenzaldehyde
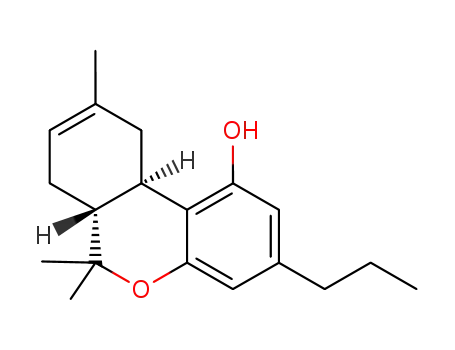
3-norpentyl-3-propyl-Δ8-tetrahydrocannabinol
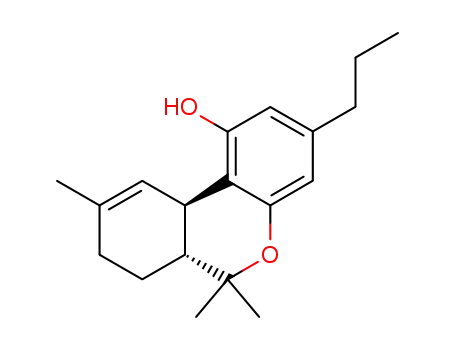
tetrahydrocannabivarin
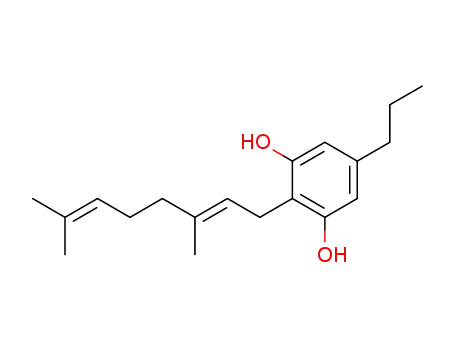
CBGV