Your Location:Home > Products > Pharmaceutical > Linagliptin
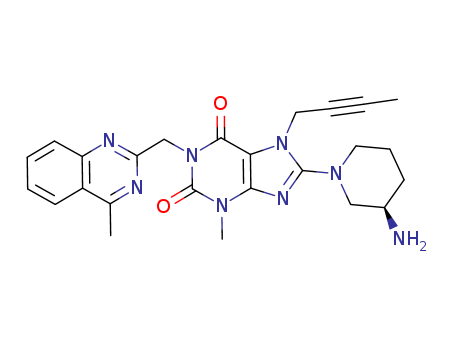

CasNo: 668270-12-0
Molecular Formula: C25H28N8O2
|
668270-12-0 Name |
|
|
Name |
Linagliptin |
|
Synonym |
linagliptin;8-[(3R)-3-Amino-1-piperidinyl]-7-(2-butynyl)-3,7-dihydro-3-methyl-1-[(4-methyl-2-quinazolinyl)methyl]-1H-purine-2,6-dione;(R)-8-(3-Amino-piperidin-1-yl)-7-but-2-ynyl-3-methyl-1-(4-methyl-quinazolin-2-ylmethyl)-3,7-dihydro-purine-2,6-dione;1H-Purine-2,6-dione, 8-((3R)-3-amino-1-piperidinyl)-7-(2-butynyl)-3,7-dihydro-3-methyl-1-((4-methyl-2-quinazolinyl)methyl)-;Bi 1356;Ondero;8-[(3R)-3-AMino-1-piperidinyl]-7-(2-butyn-1-yl)-3,7-dihydro-3-Methyl-1-[(4-Methyl-2-quinazolinyl)Methyl]-1H-purine-2,6-dione;8-[(3R)-3-AMino-1-piperidinyl]-7-(2-butynyl)-3,7-dihydro-3-Methyl-1-[(4-Methyl-2-quinazolinyl)Methyl]-1H-purine-2,6-d |
|
668270-12-0 Biological Activity |
|
|
Related Catalog |
Signaling Pathways >> Metabolic Enzyme/Protease >> Dipeptidyl Peptidase Research Areas >> Metabolic Disease |
|
Target |
IC50: 1 nM (DPP-4) |
Linagliptin (LNG) is a potent DPP- IV inhibitor, shows favorable anti-inflammatory effects in several animal model pathologies. LNG is a novel hypoglycemic drug. Linagliptin administered during morphine withdrawal significantly reduced the depressive behavior in studied mice. Linagliptin belongs to a group of new antihyperglycaemic drugs which have long half-time (T0.5 = 12 h) and linagliptin, as a selective DPP-4 inhibitor, indirectly increases the level of endogenous GLP-1 peptide.
InChI:InChI=1/C25H28N8O2/c1-4-5-13-32-21-22(29-24(32)31-12-8-9-17(26)14-31)30(3)25(35)33(23(21)34)15-20-27-16(2)18-10-6-7-11-19(18)28-20/h6-7,10-11,17H,8-9,12-15,26H2,1-3H3/t17-/m1/s1
Dipeptidyl peptidase-4 (DPP-4) inhibitors such as linagliptin are antihyperglycaemic agents with a well-established efficacy and safety profile, a low risk of hypoglycaemia and body-weight neutrality. Across the severity spectrum, linagliptin substantially reduced the hypoglycaemic burden versus glimepiride in patients with relatively early type 2 diabetes at increased cardiovascular risk.
Linagliptin (LGP) was investigated as a mild steel (MS) corrosion inhibitor in 1 M HCl solution using combined experimental and theoretical explorations. The current study is focused on the ability of Linagliptin to inhibit mild steel dissolution in 1 M HCl solution. Linagliptin is a medication mainly used together with diet and exercises in type 2 diabetes management.
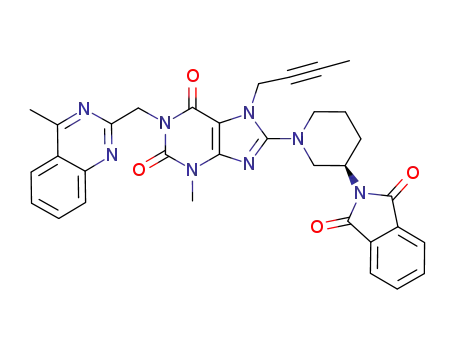
(R)-7-(but-2-yn-1-yl)-8-(3-(1,3-dioxoisoindolin-2-yl)piperidin-1-yl)-3-methyl-1-((4-methylquinazolin-2-yl)methyl)-3,7-dihydro-1H-purine2,6-dione

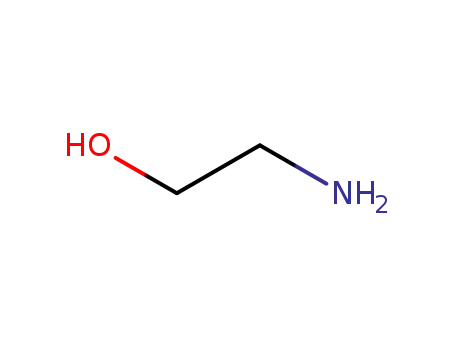
ethanolamine

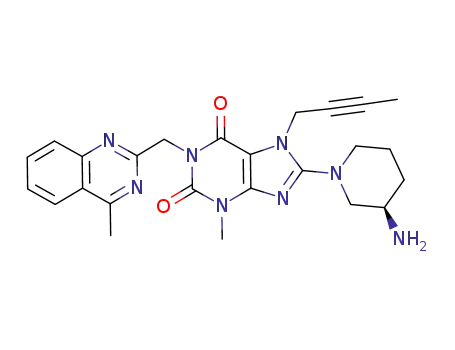
Linagliptin

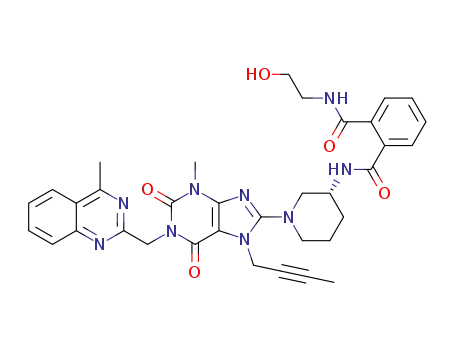
(R)-N1-(1-(7-(but-2-yn-1-yl)-3-methyl-1-((4-methylquinazolin-2-yl)methyl)-2,6-dioxo-2,3,6,7-tetrahydro-1H-purin-8-yl)piperidin-3-yl)-N2-(2-hydroxyethyl)phthalamide

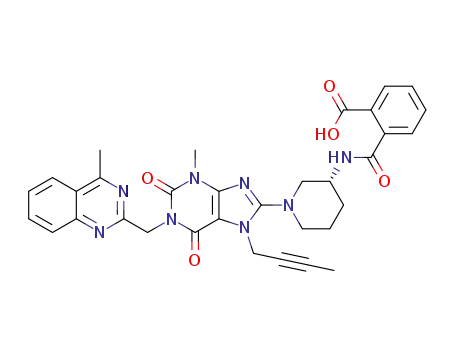
(R)-2-((1-(7-(but-2-yn-1-yl)-3-methyl-1-((4-methylquinazolin-2-yl)methyl)-2,6-dioxo-2,3,6,7-tetrahydro-1H-purin-8-yl)piperidin-3-yl)carbamoyl)benzoic acid

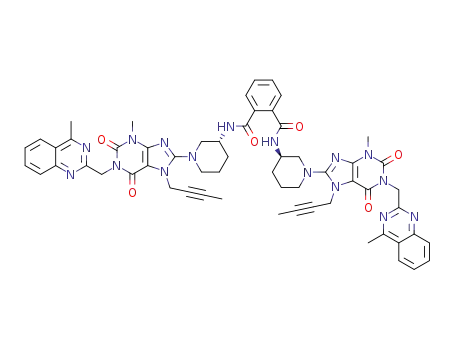
N,N'-bis((R)-1-(7-(but-2-yn-1-yl)-3-methyl-1-((4-methylquinazolin-2-yl)methyl)-2,6-dioxo-2,3,6,7-tetrahydro-1H-purin-8-yl)piperidin-3-yl)phthalamide
| Conditions | Yield |
|---|---|
|
With water; In tetrahydrofuran; at 60 ℃; for 3h;
|
81.9% |
![1-[(4-methyl-quinazolin-2-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-[(R)-3-(tert-butoxycarbonylamino)-piperidin-1-yl]-2,6-dioxo-2,3,6,7-tetrahydro-1H-purine](/upload/2023/1/b4918901-e1cf-4dd7-ae6a-754440065968.png)
1-[(4-methyl-quinazolin-2-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-[(R)-3-(tert-butoxycarbonylamino)-piperidin-1-yl]-2,6-dioxo-2,3,6,7-tetrahydro-1H-purine


Linagliptin
| Conditions | Yield |
|---|---|
|
1-[(4-methyl-quinazolin-2-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-[(R)-3-(tert-butoxycarbonylamino)-piperidin-1-yl]-2,6-dioxo-2,3,6,7-tetrahydro-1H-purine; With hydrogenchloride; In dichloromethane; water; at 20 ℃; for 3h;
With hydrogenchloride; In ethanol; water; at 35 ℃; for 2h; Temperature;
|
99.2% |
|
With methanol; water; Reflux; Inert atmosphere;
|
96.8% |
|
With methanol; water; Inert atmosphere; Reflux;
|
96.8% |
|
With methanol; water; Temperature; Reagent/catalyst; Inert atmosphere; Reflux;
|
92.8% |
|
With methanol; water; Reagent/catalyst; Reflux; Inert atmosphere;
|
92.8% |
|
With trifluoroacetic acid; In dichloromethane; at 20 ℃; for 1h;
|
91% |
|
With trifluoroacetic acid; In dichloromethane; at 5 - 20 ℃; for 5h;
|
91% |
|
With trifluoroacetic acid; In dichloromethane; at 0 - 30 ℃; for 12h;
|
90% |
|
1-[(4-methyl-quinazolin-2-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-[(R)-3-(tert-butoxycarbonylamino)-piperidin-1-yl]-2,6-dioxo-2,3,6,7-tetrahydro-1H-purine; With trifluoroacetic acid; In dichloromethane; at 20 ℃; Inert atmosphere;
With water; In 1,2-dichloro-ethane;
|
88% |
|
With trifluoroacetic acid; In dichloromethane; at 10 - 20 ℃; for 5h; Concentration; Reagent/catalyst; Solvent;
|
87.2% |
|
With formic acid; trifluoroacetic acid; at 10 - 20 ℃;
|
87.3% |
|
With trifluoroacetic acid; In dichloromethane; at 10 ℃; for 3h;
|
87.21% |
|
With trifluoroacetic acid; In dichloromethane; at 5 - 25 ℃; for 4h; Large scale;
|
82% |
|
With trifluoroacetic acid; In dichloromethane; at 1 - 15 ℃; for 20h;
|
79% |
|
1-[(4-methyl-quinazolin-2-yl)methyl]-3-methyl-7-(2-butyn-1-yl)-8-[(R)-3-(tert-butoxycarbonylamino)-piperidin-1-yl]-2,6-dioxo-2,3,6,7-tetrahydro-1H-purine; With hydrogenchloride; In methanol; water; for 4h; Reflux;
With sodium hydroxide; In methanol; water; for 5h; Reflux;
|
70% |
|
With trifluoroacetic acid; In dichloromethane; at 0 - 25 ℃; for 24h; Inert atmosphere;
|
44% |
|
With trifluoroacetic acid; In dichloromethane; water; at 20 ℃; for 3h; pH=8; Solvent; Reagent/catalyst; Temperature; Time;
|
69 g |
|
With trifluoroacetic acid; In dichloromethane; at 25 ℃; for 2h;
|
|
|
With trifluoroacetic acid; In dichloromethane; at 25 - 40 ℃;
|
|
|
With trifluoroacetic acid; In dichloromethane; at 20 ℃; for 3h;
|
2.8 g |
|
With trifluoroacetic acid; In dichloromethane; at 15 - 30 ℃; for 3h;
|
23 g |
|
With trifluoroacetic acid; In dichloromethane; for 2h;
|
|
|
With trifluoroacetic acid; In dichloromethane; at 20 ℃; for 1.5h; Concentration;
|
|
|
With trifluoroacetic acid; In dichloromethane; at 20 - 25 ℃;
|
|
|
With hydrogenchloride; In methanol; dichloromethane; at 45 ℃; for 4h;
|
4.99g |
|
With trifluoroacetic acid; In dichloromethane; at 40 - 45 ℃;
|
|
|
With zinc(II) chloride; In dichloromethane; at 30 ℃; for 5h; Temperature; Reagent/catalyst; Solvent;
|
68.6 g |
|
With trifluoroacetic acid; In dichloromethane; at 25 ℃; for 2h; Temperature; Large scale;
|
3.4 kg |
|
With trifluoroacetic acid; at 30 - 40 ℃; for 3h;
|
153.5 g |

(R)-7-(but-2-yn-1-yl)-8-(3-(1,3-dioxoisoindolin-2-yl)piperidin-1-yl)-3-methyl-1-((4-methylquinazolin-2-yl)methyl)-3,7-dihydro-1H-purine2,6-dione
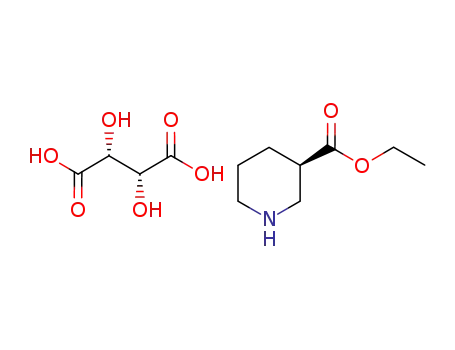
(R)-piperidine-3-carboxylic acid ethyl ester*l-tartaric acid
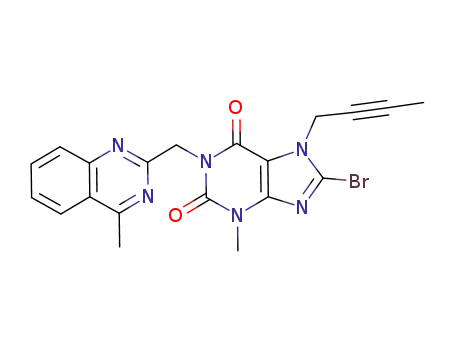
2-bromo-1-(but-2-ynyl)-4-methyl-6-((4-methylquinazolin-2-yl)methyl)-1H-imidazo[4,5-b]pyridine-5,7-(4H,6H)-dione
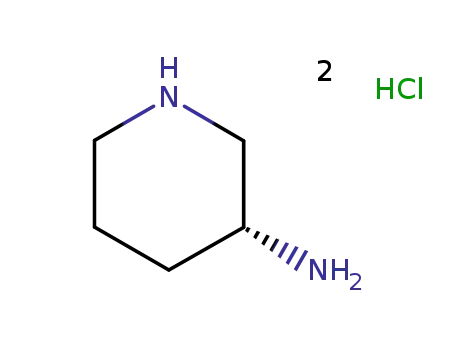
3-(R)-aminopiperidine dihydrochloride