Your Location:Home > Products > Cannabis > 2-Cyclohexen-1-ol,1-methyl-4-(1-methylethenyl)-, (1S,4R)-
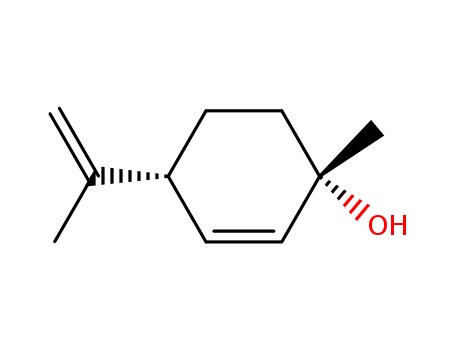

CasNo: 22972-51-6
Molecular Formula: C10H16O
Appearance: white solid
1-Methyl-4-(1-methylethenyl)-2-cyclohexen-1-ol is an acetal reagent used in the synthesis of desoxy cannabidiols and THC (T293202). 1-Methyl-4-(1-methylethenyl)-2-cyclohexen-1-ol is an acetal reagent used in the synthesis of desoxy cannabidiols and THC (T293202) related psychoactive compounds. It is formed from (+)-Limonene using a photosynthesized O2 transfer and It can inhibit the oxidation of biofilm, increase the activity of 6-phosphoglucose in biofilm, thereby protecting biofilm and stabilizing biological cell tissue.
A series of novel manganese(II)-substituted polyoxometalates, [(M(II)(H2O)3)2(WO2)2(BiW9O33)2] 10-(1), [(Mn(II)(H2O))3(SbW9O33)2]12- (2), and [(Mn(II)(H2O)3)2(Mn(II)(H2O)2)2(TeW9O33)2]8- (3), were synthesized and characterized by X-ray structure analyses. The catalytic performance is exemplified by the model substrate (R)-(+)-limonene, at ambient temperatures in a biphasic system, with excellent regioselectivities, >99%, and very high turnovers even with only a small molar excess of hydrogen peroxide.
The present disclosure relates to new cannabinoid derivatives and precursors and processes for their preparation. The disclosure also relates to pharmaceutical and analytical uses of the new cannabinoid derivatives.
The invention discloses a preparation method of (1S, 4R)-1-methyl-4-(1-methyl vinyl)-2-cyclohexene-1-alcohol, which comprises the following steps: by using D-limonene as a raw material, carrying out double bond addition and hydroxyl protection to obtain an intermediate compound; and carrying out elimination reaction and continuing deprotection to finally prepare the product.
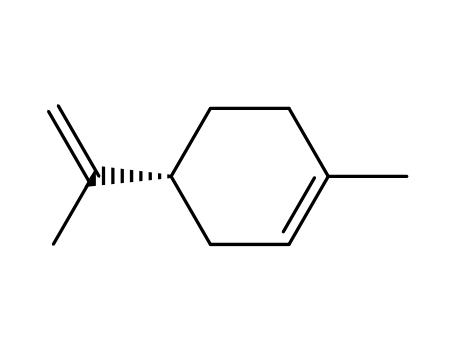
D-limonene

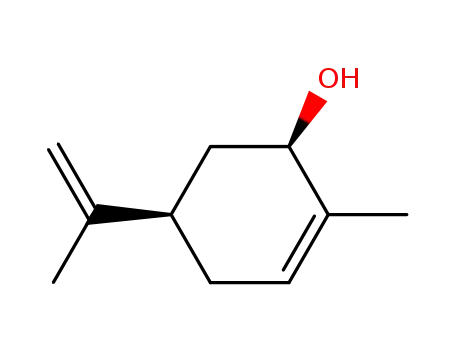
(4R,6R)-carveol

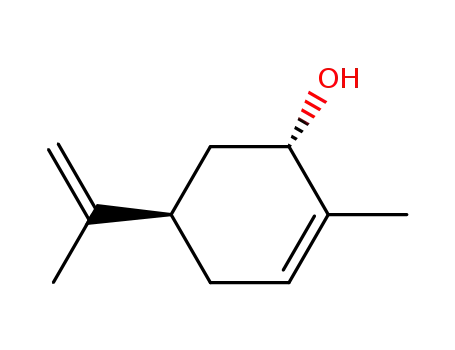
(-)-trans-carveol

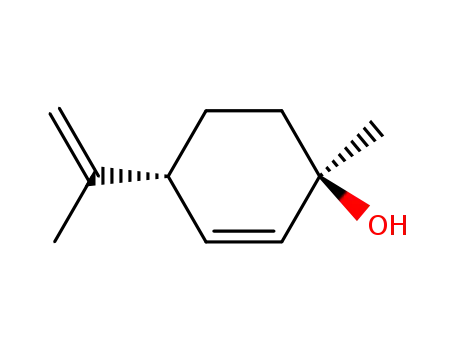
(1R,4R)-p-mentha-2,8-dien-1-ol


(1S,4R)-p-mentha-2,8-dien-1-ol

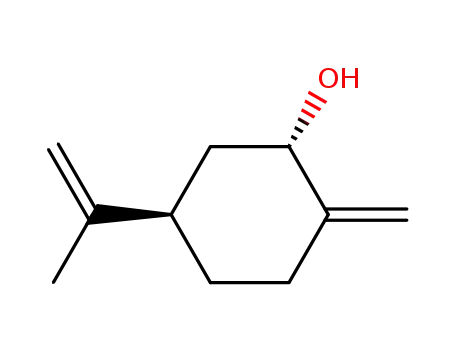
p-Menthadiene-<1(7),8>-trans-ol-(2)

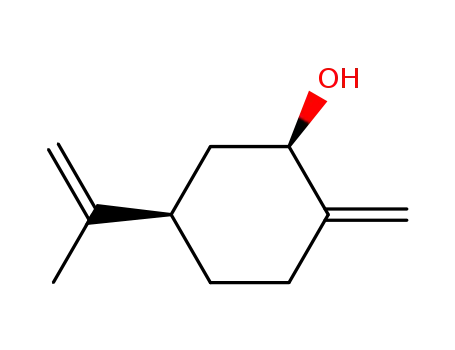
p-Menthadiene<1(7),8>-cis-ol-(2)
| Conditions | Yield |
|---|---|
|
With 1,4-diaza-bicyclo[2.2.2]octane; air; zinc 5,10,15,20-tetraphenylporphyrin; triphenylphosphine; In benzene; for 3h; Irradiation; multistep reaction: photosensitized oxygenation of olefines;
|
|
|
With sodium tetrahydroborate; tetraphenylporphyrine; oxygen; Product distribution; 1.) acetonitrile, irradiation, 2.) methanol; other sensitizers;
|
40 % Chromat. 9 % Chromat. 16 % Chromat. 7 % Chromat. 23 % Chromat. 5 % Chromat. |
|
With oxygen; fullerene-C60; In toluene; at 0 - 10 ℃; for 4h; Product distribution; Irradiation; other sensitizer in other solvent;
|
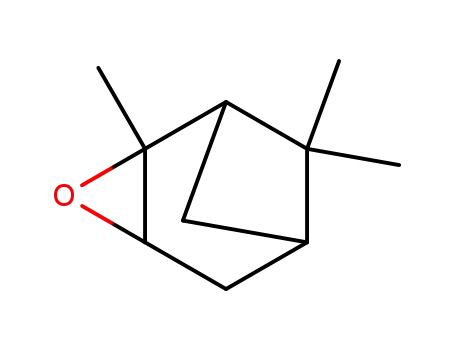
α-pinene epoxide

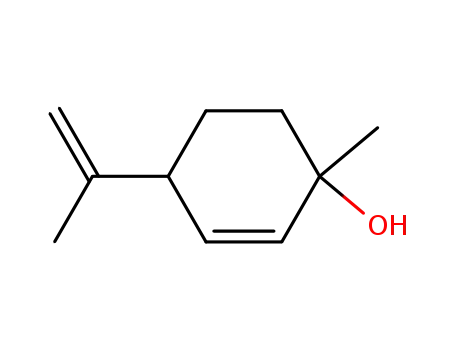
(+)-p-mentha-2,8-dien-1-ol

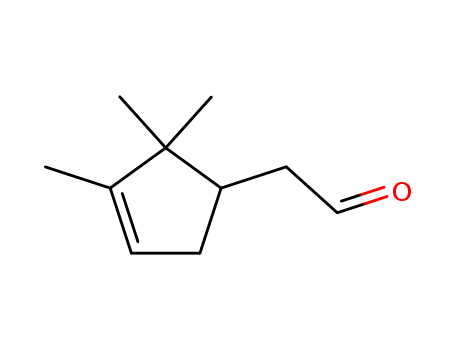
campholenyc aldehyde

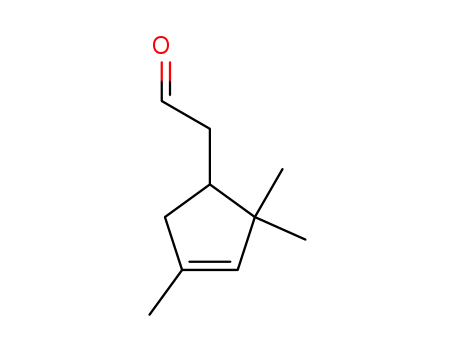
isocampholenic aldehyde

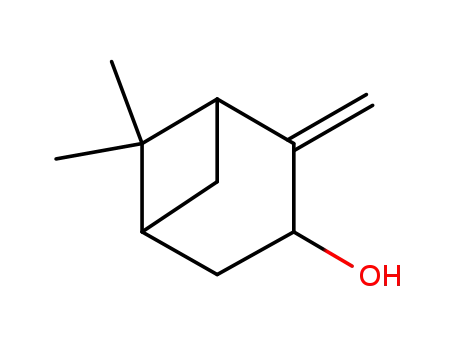
pinocarveol


(4R,6R)-carveol

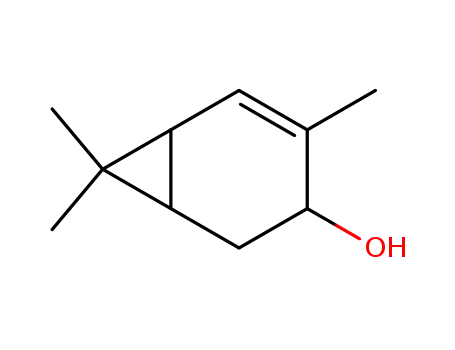
trans-2-caren-4-ol
| Conditions | Yield |
|---|---|
|
With Fe/MCM-41(N2); for 0.166667h;
|
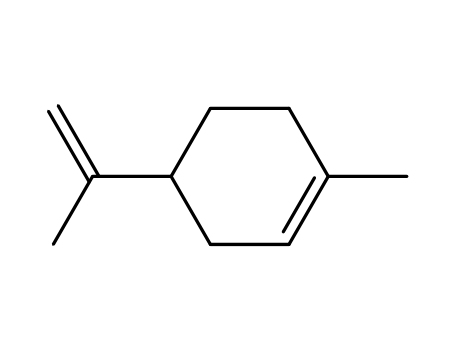
limonene.

α-pinene epoxide

D-limonene
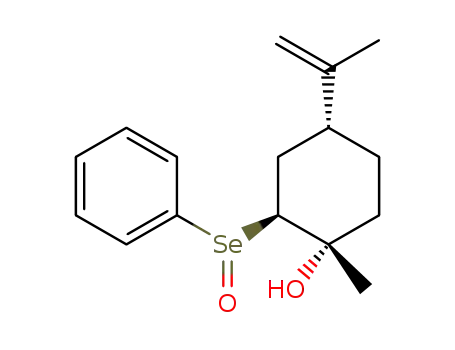
(1S,2S,4R)-2-phenylseleninyl-p-menth-8-en-1-ol
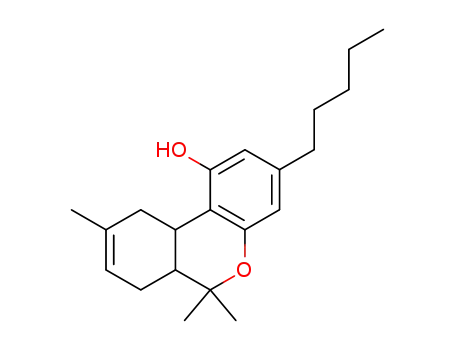
6,6,9-Trimethyl-3-pentyl-6a,7,10,10a-tetrahydro-6H-benzo[c]chromen-1-ol
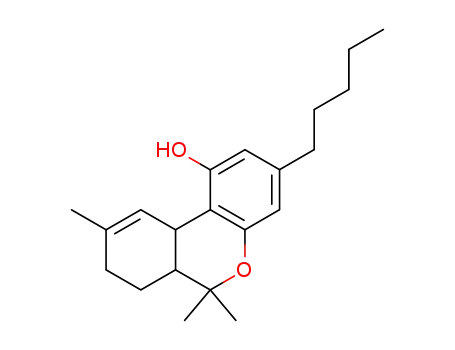
tetrahydrocannabinol
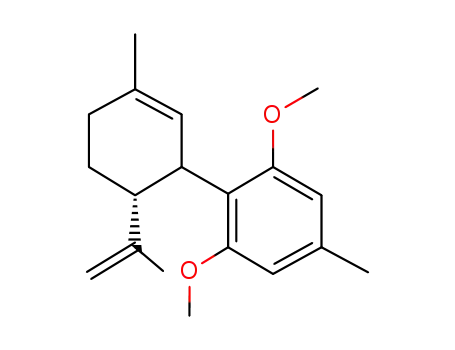
abn-cannabidiol
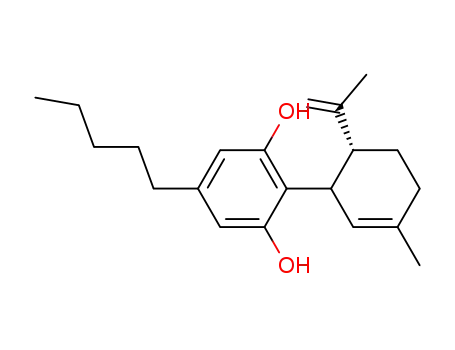
cannabidiol