Your Location:Home > Products > Pharmaceutical > tert-butylmagnaeium chloride
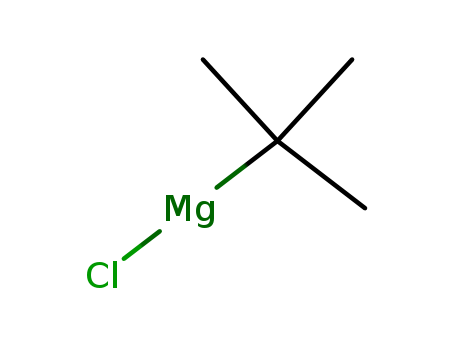

CasNo: 677-22-5
Molecular Formula: C4H9ClMg
Appearance: clear faint grey to grey, grey-brown or light
|
677-22-5 Name |
|
|
Name |
tert-butylmagnaeium chloride |
|
Synonym |
tert-Butylmagnesium chloride, 1M in MeTHF;tert-Butylmagnesium chloride [1.0 M solution in THF];tert-Butylmagnesium chloride, 1,7M solution in diethyl ether, AcroSeal§3;tert-Butylmagnesium chloride, 1.7M solution in THF, AcroSeal;tert-Butylmagnesium chloride, 1.7M solution in diethyl ether, AcroSeal;tert-ButylMagnesiuM chloride ,1.0 M in tetrahydrofuran;tert-butylMagnesiuM;(1,1-Dimethylethyl)magnesium chloride |
|
677-22-5 Chemical & Physical Properties |
|
|
Melting point |
46ºC to 50ºC |
|
Density |
0.931 g/mL at 25 °C |
|
Molecular Formula |
C4H9ClMg |
|
Molecular Weight |
116.87200 |
|
Flash Point |
34 °F |
|
LogP |
2.44360 |
|
Exact Mass |
116.02400 |
|
Storage condition |
2-8°C |
Tert-butylmagnesium chloride (tBuMgC1) is a Grignard reagent used in organic synthesis. It is also involved in copper-catalyzed cross-coupling reaction with primary-alkyl halides. tert-Butylmagnesium chloride could even be used as the initial nucleophile to afford the highly sterically hindered S-tert-butyl sulfoximine 2v in 53% yield; such products have previously been prepared by three sequential deprotonations/methylations of an S-methyl sulfoximine.
InChI:InChI=1/C4H9.ClH.Mg/c1-4(2)3;;/h1-3H3;1H;/q;;+1/p-1/rC4H9Mg.ClH/c1-4(2,3)5;/h1-3H3;1H/q+1;/p-1
The earlier results concerning the kinetics of polymerization and initiator consumption, molecular weight distribution, and the nature of the chain ends in the polymerization of vinyl chloride initiated by tert-butylmagnesium chloride (tBuMgCl) in tetrahydrofuran and other solvents are rediscussed in terms of the simultaneous participation of an ionic mechanism and a radical mechanism.
In the study of the dehydration of di-tert-butylmethyl alcohol to give trimethylethylene through elimination of a tert-butyl group (as isobutylene), Crowded carbonyl compounds when reacted with tert-butyllithium or tert-butylmagnesium chloride followed by thionyl chloridetreatment give in a one-pot reaction olefins.

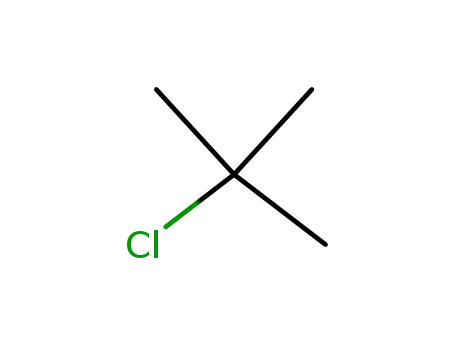
tertiary butyl chloride

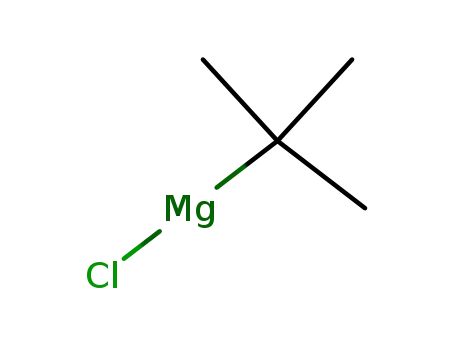
tert-butylmagnesium chloride
| Conditions | Yield |
|---|---|
|
|

tertiary butyl chloride

magnesium


tert-butylmagnesium chloride
| Conditions | Yield |
|---|---|
|
In tetrahydrofuran;
|
|
|
iodine; In tetrahydrofuran; diethyl ether; at 25 - 40 ℃;
|

tertiary butyl chloride
9,10-dihydro-9,10-anthracendiyl-tris(THF)magnesium
n-propyltrichlorosilane
magnesium
3,3-dimethylbutanol
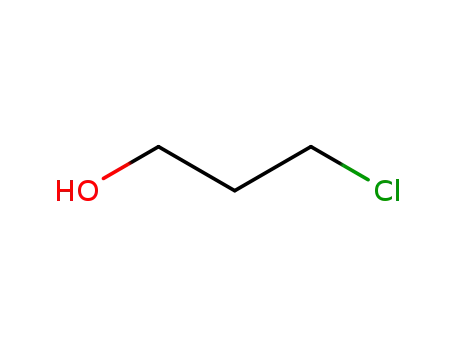
1-chloro-3-hydroxypropane
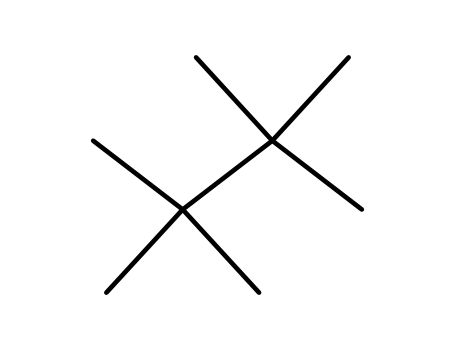
tetramethyl-2,2,3,3 butane
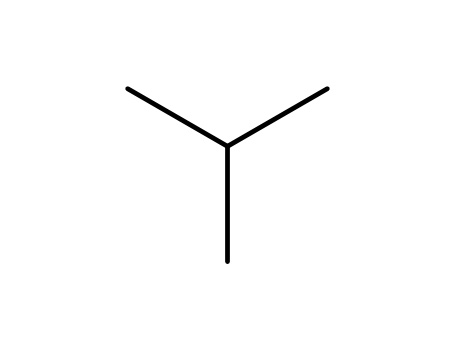
Isobutane