Your Location:Home > Products > Fine Chemicals > THIOPHEN-2-YL-MAGNESIUM BROMIDE 1.0M
CasNo: 5713-61-1
Molecular Formula: C4H3BrMgS
|
5713-61-1 Name |
|
|
Name |
2-Thienylmagnesium bromide |
|
Synonym |
2-ThienylMagnesiuM broMide, 1.0 M solution in THF, SpcSeal;magnesium,2H-thiophen-2-ide,bromide;Bromo-2-thienylmagnesium;THIOPHEN-2-YL-MAGNESIUM BROMIDE 1.0M;2-Thienylmagnesium bromide solution, 1.0M in tetrahydrofuran;thiophen-2-yl-magnesium bromide solution;2-Thienylmagnesium bromide solution;2-Thienylmagnesium bromide, 1M solution in THF, AcroSeal |
|
5713-61-1 Chemical & Physical Properties |
|
|
Boiling point |
65ºC |
|
Density |
1.011 g/mL at 25ºC |
|
Molecular Formula |
C4H3BrMgS |
|
Molecular Weight |
187.34100 |
|
Flash Point |
-6 °F |
|
PSA |
28.24000 |
|
LogP |
2.39390 |
|
Exact Mass |
185.89900 |
|
Appearance of Characters |
Solution | Yellow to orange to brown |
2-Thienylmagnesium bromide is Yellow to orange to brown liquid, which is a Grignard reagent that can be used as a reactant to synthesize: 1-(2-thienyl)-carbinols by condensation reaction with aldehydes. 2-Thienylmagnesium bromide was treated with epichlorohydrin to yield 1-(2-thieny1)-3-chloro-2-propanol (VI). Grignard reaction between 2-thienylmagnesium bromide and nitrile groups can used to produce a thiophene-functionalized polystyrene macromonomer (ThPStM).
InChI:InChI=1/C4H3S.BrH.Mg/c1-2-4-5-3-1;;/h1-3H;1H;/q-1;;+2/p-1
Magnesium is known to form typical Grignard reagents with 2-iodothiophene and 2- bromothio-phene.2-Chlorothiophene is relatively inactive with. magnesium. Accordingly, 2-thienylmagne-sium bromide was used for this study.
A reagent scope for the ring-opening reaction revealed that reactions with less-sterically hindered aryl Grignard reagents, such as phenyl and para-substituted phenylmagnesium bromides, provided the dipyrrin products. In contrast, no reaction occurred, and unreacted NiDOP was recovered when alkyl, ethynyl, and 2-thienylmagnesium bromides and sterically hindered mesitylmagnesium bromide were used for the reactions.
2-bromothiophene

![1-[1-(3-hydroxymethyl-piperidino)-cyclohexan-1-yl]-carbonitrile](/upload/2023/1/fc98d0fd-229d-46a1-b8ae-fd9c1031b891.png)
1-[1-(3-hydroxymethyl-piperidino)-cyclohexan-1-yl]-carbonitrile

thiophen-2-yl magnesium bromide

![N-[1-(thien-2-yl)-cyclohexan-1-yl]-3-hydroxymethyl-piperidine](/upload/2023/1/b6296caf-5d3a-4485-8b9b-cefb17460aba.png)
N-[1-(thien-2-yl)-cyclohexan-1-yl]-3-hydroxymethyl-piperidine
| Conditions | Yield |
|---|---|
|
With magnesium;
|

2-bromothiophene


thiophen-2-yl magnesium bromide
| Conditions | Yield |
|---|---|
|
With magnesium; In tetrahydrofuran; at 20 ℃; for 0.166667h; Inert atmosphere; Flow reactor;
|
93% |
|
With magnesium; In diethyl ether;
|
|
|
With magnesium;
|
|
|
With magnesium;
|
|
|
With magnesium; In tetrahydrofuran; at 80 ℃; for 0.25h; microwave irradiation;
|
|
|
With magnesium; In tetrahydrofuran; at 0 - 20 ℃; for 3.33333h;
|
|
|
With magnesium; In tetrahydrofuran;
|
|
|
With magnesium;
|
|
|
With ammonium hydroxide; ammonium chloride; magnesium; In cyclohexanone;
|
|
|
With magnesium; In tetrahydrofuran;
|
|
|
With magnesium; iodine; In diethyl ether; for 1.33333h; Heating / reflux;
|
|
|
With 1,2-dibromobutane; magnesium; In tetrahydrofuran; at 50 - 65 ℃; Inert atmosphere;
|
|
|
With magnesium; In tetrahydrofuran; at 20 ℃; for 1.5h;
|
|
|
With iodine; magnesium; In tetrahydrofuran; for 3h; Inert atmosphere; Reflux;
|
|
|
With diisobutylaluminium hydride; magnesium; lithium chloride; In tetrahydrofuran; at 20 ℃; Inert atmosphere; Schlenk technique;
|
thiophene
2-thienyl lithium

2-bromothiophene
oxothien-2-yl-acetic acid 1-azabicyclo[2.2.2]oct-4-yl ester
2-thiophenethanol
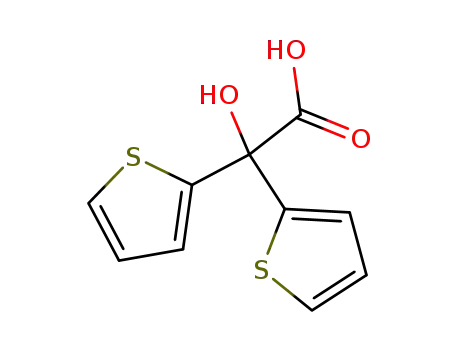
2-hydroxy-2,2-di(thiophen-2-yl)acetic acid
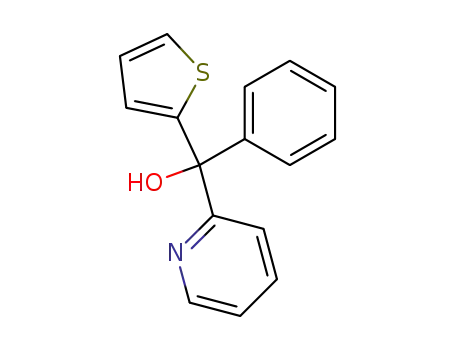
Phenyl-[2]pyridyl-[2]thienyl-methanol
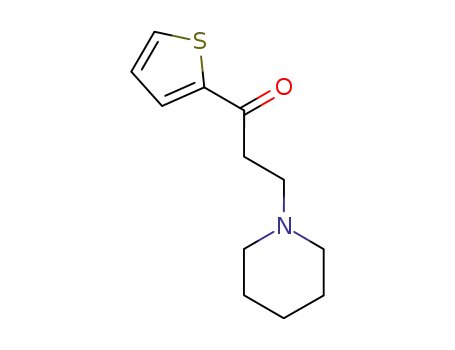
3-(piperidin-1-yl)-1-(thiophen-2-yl)propan-1-one