Your Location:Home > Products > Pharmaceutical > Cyclopropylboronic acid
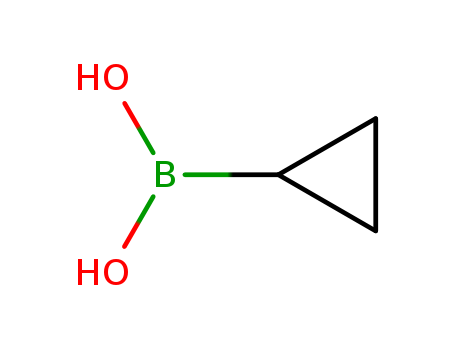

CasNo: 411235-57-9
Molecular Formula: C3H7BO2
Appearance: white powder
|
411235-57-9 Name |
|
|
Name |
Cyclopropylboronic acid |
|
Synonym |
AKOS BRN-0457;CYCLOPROPYLBORONIC ACID;Cyclopropylboronic acid,monohydrate;CYCLOPROPYLBORONIC ACID,MONOHYDRATE;BORONIC ACID, CYCLOPROPYL- (9CI);;Cyclopropylboronic acid (contains varying amounts of anhydride);CYCLOROPYLBORONIC ACID;Boronic acid, cyclopropyl- (9CI);Cyclopropylboronic |
|
411235-57-9 Chemical & Physical Properties |
|
|
Melting point |
90-95 °C(lit.) |
|
Boiling point |
205.1±23.0 °C at 760 mmHg |
|
Density |
1.1±0.1 g/cm3 |
|
Molecular Formula |
C3H7BO2 |
|
Molecular Weight |
85.897 |
|
Flash Point |
77.9±22.6 °C |
|
PSA |
40.46000 |
|
LogP |
0.20 |
|
Exact Mass |
86.053909 |
|
Vapour Pressure |
0.1±0.9 mmHg at 25°C |
|
Index of Refraction |
1.443 |
|
Storage condition |
Store below -20 |
Cyclopropylboronic acid is White to off-white powder, which is an organoboronic acid commonly used in highly efficient Suzuki coupling reactions. Cyclopropylboronic acid has been elegantly used as a cyclopropylating reagent in N-cyclopropylation reactions.
Uses
suzuki reaction
InChI:InChI=1/C3H7BO2/c5-4(6)3-1-2-3/h3,5-6H,1-2H2
Terminal acetylenes undergo a Schwartz’s Reagent catalysed hydroboration; subsequent addition of further Schwartz’s Reagent and Lewis acid mediated activation of neighbouring silyl ether, allows cyclisation to access a range of cyclopropyl boronic acid pinacol esters. The scope includes aromatic, aliphatic, quaternary and spiro substituted cyclopropyl rings which can be transformed via Suzuki coupling into a range of lead-like substituted cyclopropyl aryl products.
Use of novel cyclopropylboronic reagents The synthesis of novel cyclopropylboronic reagents … In 2002, such a method was applied even for the parent cyclopropylboronic acid 12.
The invention discloses a synthesis method of cyclopropyl boric acid, and belongs to the field of boric acid synthesis in organic chemistry. The synthesis method disclosed by the invention is simple in operation, the use of cyclopropyl bromide in a traditional technological method is avoided by adopting cyclopropanation reaction under metal catalysis, and a new synthesis path is provided for synthesis of the cyclopropyl boric acid.

diazomethane

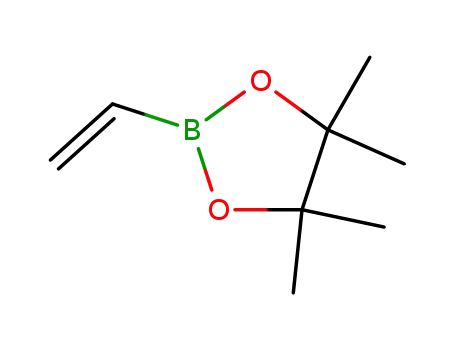
pinacol vinylboronate

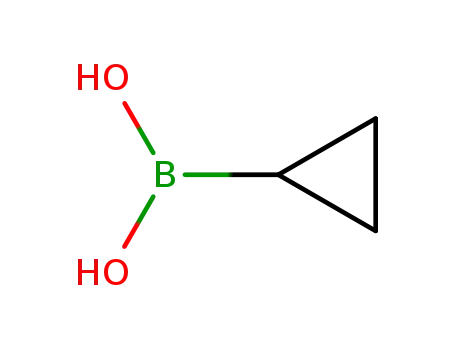
cyclopropylboronic acid
| Conditions | Yield |
|---|---|
|
diazomethane; pinacol vinylboronate; With palladium diacetate; In tert-butyl methyl ether; Inert atmosphere;
With sodium periodate; In tetrahydrofuran; water; at 20 ℃; for 0.05h; Reagent/catalyst; Temperature;
|
86% |
ethene

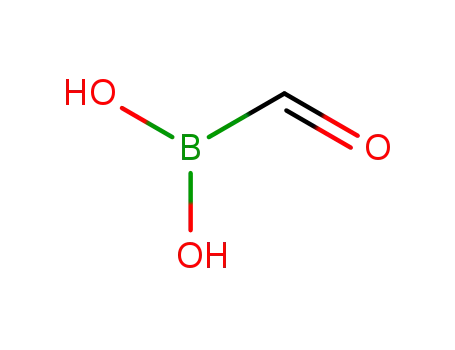
formyl boronic acid

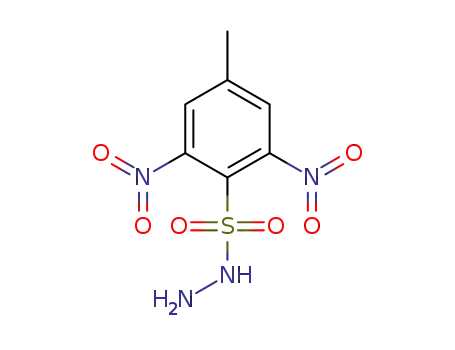
3,5-dinitro-p-toluenesulfonylhydrazide


cyclopropylboronic acid
| Conditions | Yield |
|---|---|
|
formyl boronic acid; 3,5-dinitro-p-toluenesulfonylhydrazide; In methanol; Reflux;
With n-butyllithium; In tetrahydrofuran; at 0 ℃; for 0.5h;
ethene; Reagent/catalyst; Solvent; Further stages;
|
92% |
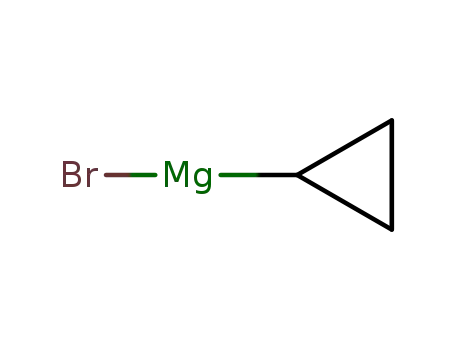
cyclopropylmagnesium bromide
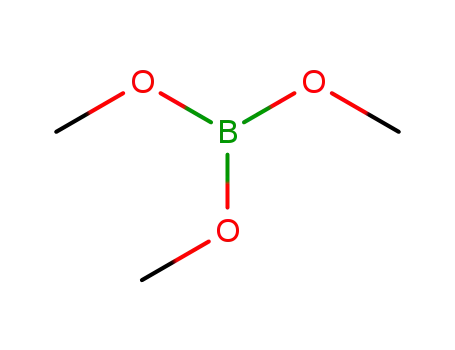
Trimethyl borate
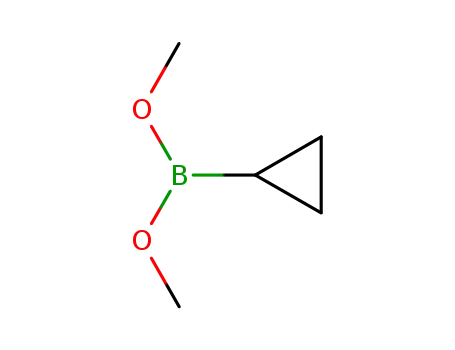
C5H11BO2
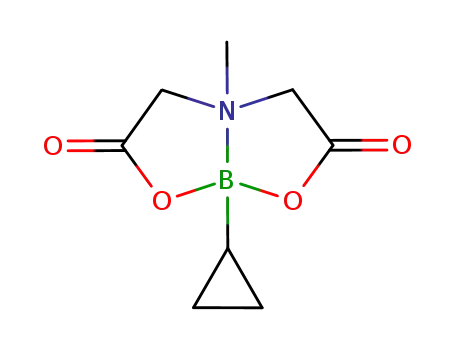
cyclopropylboronic acid methyliminodiacetic acid ester
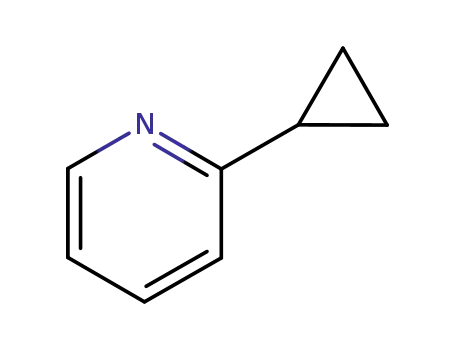
2-cyclopropyl pyridine
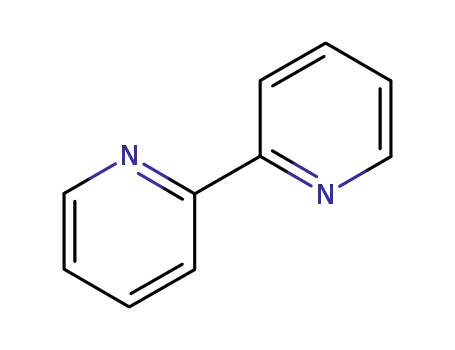
[2,2]bipyridinyl
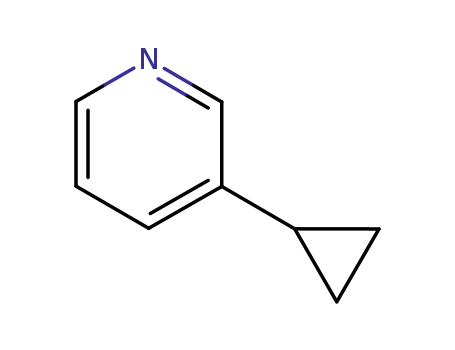
3-cyclopropylpyridine
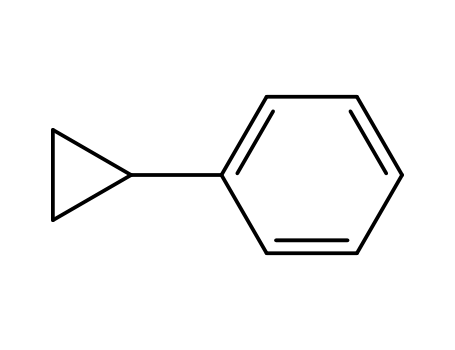
cyclopropylbenzene