Your Location:Home > Products > Pharmaceutical > Indium chloride (InCl3)
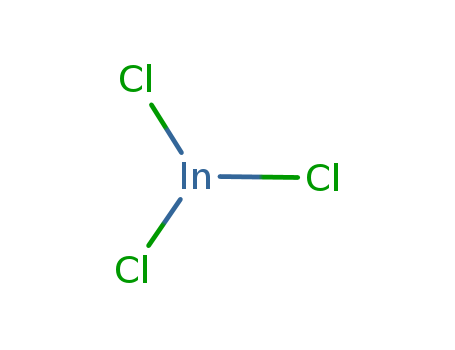

CasNo: 10025-82-8
Molecular Formula: InCl3
Appearance: white crystalline powder
|
10025-82-8 Name |
|
|
Name |
Indium chloride |
|
Synonym |
InCl3;Indium chloride (InCl3);indiumchloride(incl3);INDIUM CHLORIDE;Indium(III) chloride, anhydrous (metals basis);Indium(III) chloride ,99.995% [trace metal basis];Indium(III) chloride, synthesis grade;"Indium (III) chloride, anhydrous/ 99.99%" |
|
10025-82-8 Chemical & Physical Properties |
|
|
Melting point |
262 °C (dec.)(lit.) |
|
Boiling point |
300°C |
|
Density |
3.46 g/mL at 25 °C(lit.) |
|
Molecular Formula |
Cl3Ho |
|
Molecular Weight |
271.28900 |
|
Flash Point |
300°C subl. |
|
LogP |
2.06850 |
|
Exact Mass |
269.83700 |
|
Water Solubility |
reacts |
Indium chloride is a useful catalyst for aqueous organic reactions including C-C bond formation, aldol reaction, Friedel-Crafts acylation, Diels-Alder reaction and reduction1-4. In electroplating using a solution of the salt with dextrose and NaCN. The indium chloride compound could be evaporated, followed by condensation. Indium chloride (InCl3) is a potential additive to develop aqueous Mg batteries with high performance.
InChI:InChI=1/3ClH.In.H2O/h3*1H;;1H2/q;;;+3;/p-3
We demonstrate that alloying Sb3+ into chiral indium-chloride hybrids dramatically increases the photoluminescence quantum yield in two new series of chiral indium-antimony chlorides.
The catalyst, indium chloride, exhibits high catalytic activity with a variety of toluene derivatives in continuous flow. Good yields (59–77%) were obtained in all the cases. Improved selectivity was observed under flow conditions, when compared to batch operation.
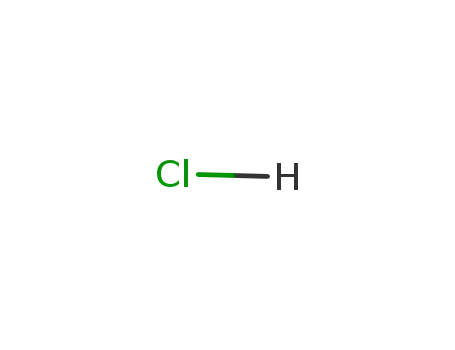
hydrogenchloride

indium

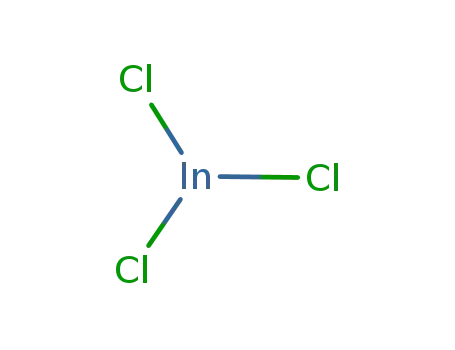
indium(III) chloride
| Conditions | Yield |
|---|---|
|
With water; starting with slightly wettened In without heating, then at 120 to 140°C, by adding more water;
|
|
|
byproducts: H2; wet HCl gas;
|
|
|
In hydrogenchloride; prepn. by dissolving In in HCl according to S. V. Bleshinskii, V. F. Abramova, Chemistry of Indium (Akad. Nauk Kirg. SSR, Frunze, 1958);
|
|
|
In hydrogenchloride;
|
|
|
byproducts: H2; wet HCl gas;
|
|
|
In hydrogenchloride; metallic indium dissoln. in concd. hydrochloric acid; soln. evapn. (quartz dish) until ppt. formation, ppt. transferring to quartz tube, sublimation at 600°C;
|
|
|
In hydrogenchloride; indium dissolved in aq. HCl;
|
indium

chlorine


indium(III) chloride
| Conditions | Yield |
|---|---|
|
in dry Cl2-flow;
|
|
|
Glowing to red heat, yellow-green flame.;
|
|
|
air removed by N2-flow, then reaction at 600°C in N2/Cl2-flow;
|
|
|
|
|
|
In solid; other Radiation; placing metal in evacuated flask; filling with reactive gas; introducing in microwave cavity; reacting; detn. by X-ray powder diffraction;
|
|
|
In neat (no solvent); indium heated at 200°C under stream of Cl2 and N2; heated to 450°C (Cl2 shut off); sublimation;
|
|
|
In neat (no solvent); chlorine gas passed over In at 450°C;
|
|
|
Glowing to red heat, yellow-green flame.;
|
|
|
In neat (no solvent); at 520°C;
|
|
|
purified by distn.;
|
|
|
In neat (no solvent); Cl2-stream;
|
|
|
indium with sublimed InCl3 chlorination in chlorine stream at 600°C; differential thermal anal., X-ray diffraction;
|
|
|
In neat (no solvent); Cl2 passed over In at temp. about 450 K; elem. anal.;
|
sulfur dioxide

chlorine
thionyl chloride
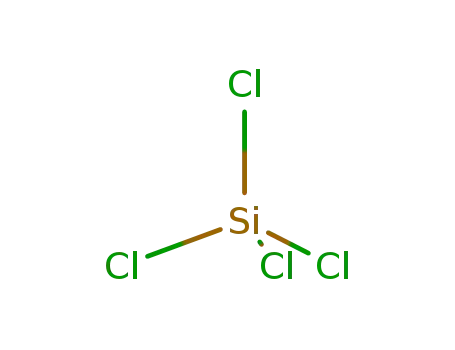
tetrachlorosilane
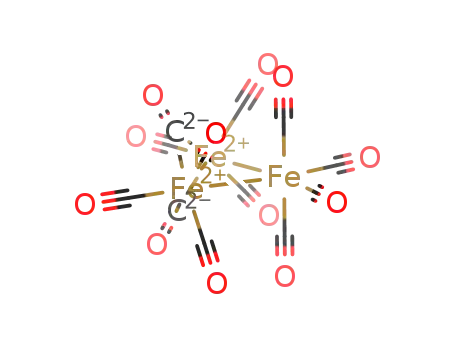
triiron dodecarbonyl

indium chloride
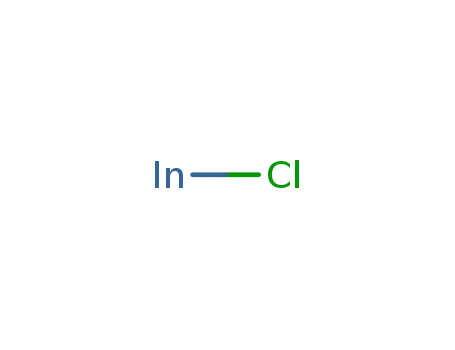
indium monochloride
indium