Your Location:Home > Products > Cannabis > Divaric acid
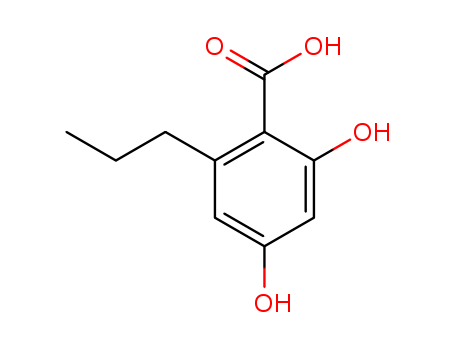

CasNo: 4707-50-0
Molecular Formula: C10H12O4
|
4707-50-0 Name |
|
|
Name |
Varinolic acid |
|
Synonym |
2,4-Dihydroxy-6-propylbenzoic acid;Divaric acid;Varinolic Acid;Benzoic acid, 2,4-dihydroxy-6-propyl- |
|
4707-50-0 Chemical & Physical Properties |
|
|
Melting point |
169 °C (decomp) |
|
Boiling point |
385.8ºC at 760mmHg |
|
Density |
1.324g/cm3 |
|
Molecular Formula |
C10H12O4 |
|
Molecular Weight |
196.20000 |
|
Flash Point |
201.3ºC |
|
PSA |
77.76000 |
|
LogP |
1.74850 |
|
Exact Mass |
196.07400 |
|
Index of Refraction |
1.606 |
Varinolic acid is an analytical reference standard categorized as an intermediate in the phytocannabinoid biosynthetic pathway. Divarinic Acid is an antibacterial compound that strongly inhibits bacterial growth of Bacillus subtilis, Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa.
InChI:InChI=1/C10H12O4/c1-2-3-6-4-7(11)5-8(12)9(6)10(13)14/h4-5,11-12H,2-3H2,1H3,(H,13,14)
Prenyltransferase, also known as cannabigerolic acid synthase (CBGAS), produces cannabigerolic acid (CBGA) and cannabigerovarinic acid (CBGVA), which are derived from C21 and C19 precursors formed from olivetolic and divarinic acids, respectively. Biosynthesis pathway of cannabinoids. Hexanoyl-CoA and butyl-CoA are used as substrates to generate olivetolic and divarinic acid.
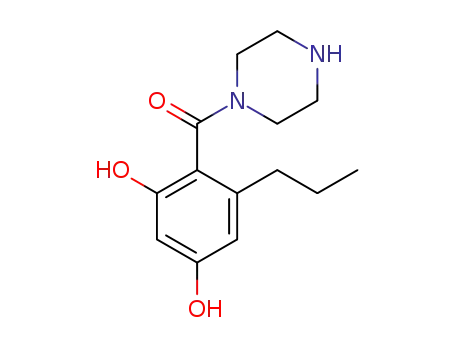
5-propyl-4-(piperazine-1-carbonyl)-1,3-diol

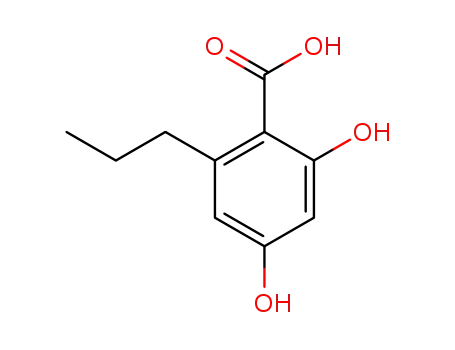
2,4-dihydroxy-6-propylbenzoic acid
| Conditions | Yield |
|---|---|
|
With potassium tert-butylate; In water; at 20 ℃;
|
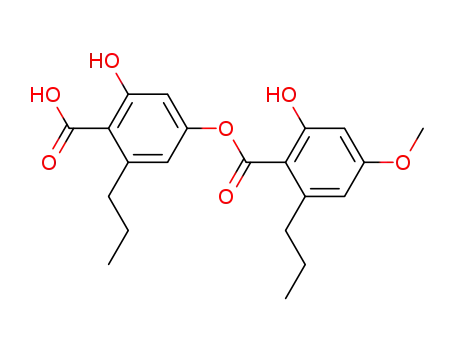
divaricatic acid


2,4-dihydroxy-6-propylbenzoic acid
| Conditions | Yield |
|---|---|
|
With potassium hydroxide;
|
|
|
Multi-step reaction with 2 steps
1: 1.5 g / SO2Cl2 / diethyl ether / 24 h / Ambient temperature
2: 60 mg / H2SO4 / 0.25 h / 0 °C
With sulfuryl dichloride; sulfuric acid; In diethyl ether;
|

divaricatic acid
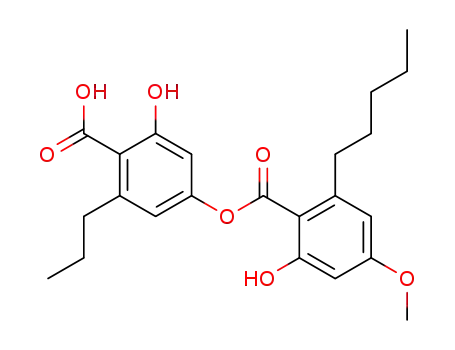
2-hydroxy-4-(2-hydroxy-4-methoxy-6-pentyl-benzoyloxy)-6-propyl-benzoic acid
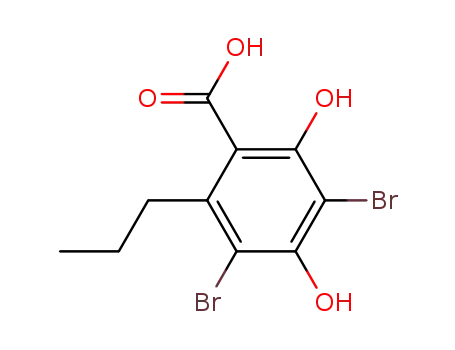
3,5-dibromo-2,4-dihydroxy-6-propyl-benzoic acid
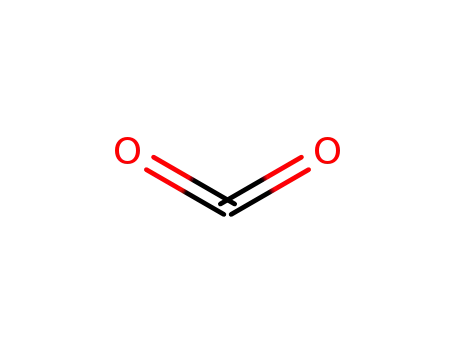
carbon dioxide
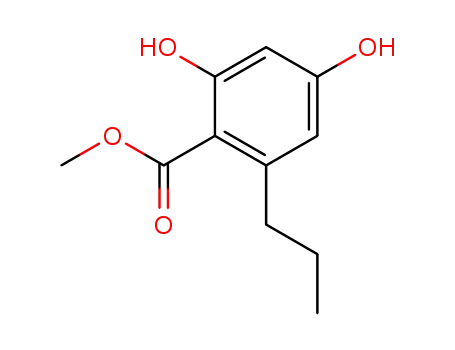
2,4-dihydroxy-6-n-propylbenzoic acid, methyl ester
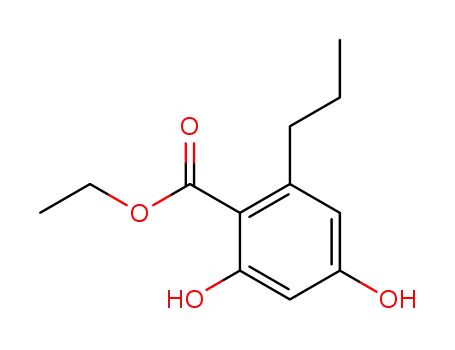
2,4-dihydroxy-6-propylbenzoic acid ethyl ester
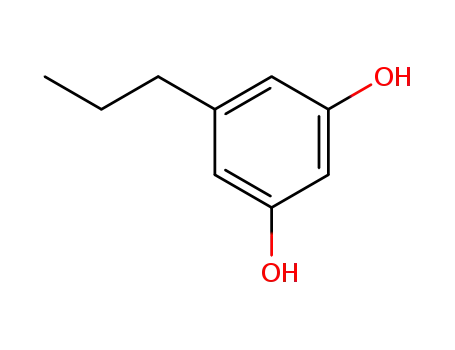
5-propyl-1,3-benzenediol