Your Location:Home > Products > Cannabis > Cannabigerol
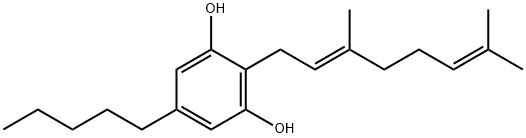

CasNo: 25654-31-3
Molecular Formula: C21H32 O2
|
25654-31-3 Name |
|
|
Name |
Cannabigerol |
|
Synonym |
cannabigerol;Cannabigerol (exempt preparation);Cannabigerol solution;2-[(2E)-3,7-dimethylocta-2,6-dienyl]-5-pentylbenzene-1,3-diol;Cannabigerol (CRM);2-[(2E)-3,7-Dimethyl-2,6-octadienyl]-5-pentylresorcinol;2-[(E)-3,7-Dimethyl-2,6-octadienyl]-5-pentyl-1,3-benzenediol;1,3-Benzenediol, 2-[(2E)-3,7-diMethyl-2,6-octadien-1-yl]-5-pentyl- |
|
25654-31-3 Chemical & Physical Properties |
|
|
Melting point |
49-50 °C |
|
Boiling point |
470.4±40.0 °C at 760 mmHg |
|
Density |
1.0±0.1 g/cm3 |
|
Molecular Formula |
C21H32O2 |
|
Molecular Weight |
316.478 |
|
Flash Point |
207.2±21.9 °C |
|
PSA |
40.46000 |
|
LogP |
7.47 |
|
Exact Mass |
316.240234 |
|
Vapour Pressure |
0.0±1.2 mmHg at 25°C |
|
Index of Refraction |
1.536 |
|
Storage condition |
-20°C |
Cannabigerol (CBG) is one of the major phytocannabinoids present in Cannabis sativa L. It is a cannabinoid from the plant Cannabis sativa that lacks psychotomimetic effects. Research on cannabigerol (CBG) mainly due to its low concentration in the Cannabis plant.
InChI:InChI=1/C21H32O2/c1-5-6-7-11-18-14-20(22)19(21(23)15-18)13-12-17(4)10-8-9-16(2)3/h9,12,14-15,22-23H,5-8,10-11,13H2,1-4H3/b17-12+
Cannabidiol (CBD) and cannabigerol (CBG) are the two main non-psychotropic phytocannabinoids with high application potential in drug development. In CBG, there is an extra dimethyloctadienyl structural pattern, which is most likely responsible for the disruption to the redox status and hepatic environment. In this study, we focused on in vivo safety evaluation and the effect of CBD and CBG on redox status in rats in a 90-d experiment.
Cannabigerol (CBG) is a nonpsychoactive ingredient that was extracted in the same year with THC . CBG has been suggested as an antinociceptive compound, but the antinociceptive activity of CBG has been evaluated in a few preclinical studies. In this study, we demonstrated the antinociceptive activity of CBG in different pain models and elucidated the mechanism by which CBG targets two receptors, TRPV1 and CB2R, to produce antinociceptive effects.
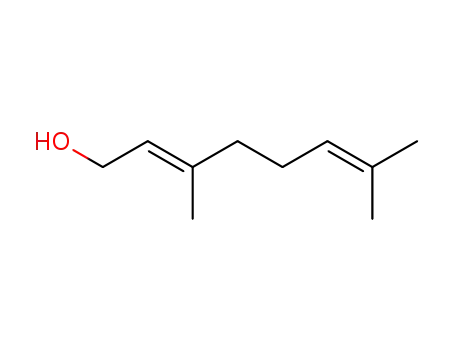
Geraniol

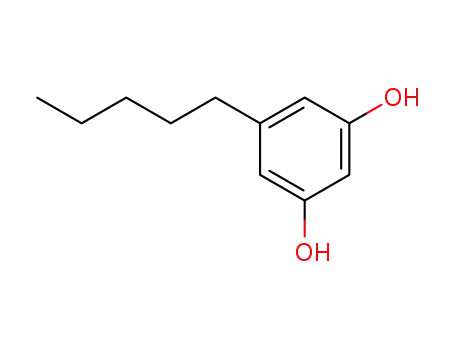
Olivetol

![2-[(2E)-3,7-dimethylocta-2,6-dienyl]-5-pentyl-benzene-1,3-diol](/upload/2023/1/d3368536-f417-4bbc-ad56-9fbbdb8e334a.png)
2-[(2E)-3,7-dimethylocta-2,6-dienyl]-5-pentyl-benzene-1,3-diol
| Conditions | Yield |
|---|---|
|
With toluene-4-sulfonic acid; In chloroform; at 20 ℃; for 12h; Darkness;
|
80% |
|
With aluminum oxide; In 1,2-dichloro-ethane; for 6h; Time; Reflux;
|
62% |
|
With toluene-4-sulfonic acid; In chloroform; at 20 ℃; for 12h; Darkness;
|
39.9% |
|
With toluene-4-sulfonic acid; In chloroform; at 20 ℃; for 12h; Darkness;
|
39.9% |
|
With toluene-4-sulfonic acid; In chloroform; at 20 ℃; for 12h; Darkness;
|
39.9% |
|
With toluene-4-sulfonic acid; In chloroform; at 20 ℃; for 12h; Darkness;
|
39.9% |
|
With boron trifluoride diethyl etherate; silica gel; In dichloromethane; for 48h; Ambient temperature;
|
29% |
|
With toluene-4-sulfonic acid; In chloroform; at 20 ℃; for 12h; Darkness;
|
28% |
|
With toluene-4-sulfonic acid; In chloroform; at 20 ℃; for 14h; Reagent/catalyst; Temperature;
|
25% |
|
With toluene-4-sulfonic acid; In toluene; at 10 ℃; for 72h; Reagent/catalyst; Temperature; Solvent; Darkness;
|
13% |
|
With aluminum oxide; In hexane; for 6h; Solvent; Reagent/catalyst; Reflux;
|
12 %Spectr. |
|
With toluene-4-sulfonic acid; In chloroform; at 20 ℃; for 12h; Solvent; Temperature; Darkness;
|
1.4 g |
![(E)-8-(3,7-dimethylocta-2,6-dien-1-yl)-7-hydroxy-2,2-dimethyl-5-pentyl-4H-benzo[d][1,3]dioxin-4-one](/upload/2023/1/cc2a6f51-9c9f-480a-91a4-8a0b14dca766.png)
(E)-8-(3,7-dimethylocta-2,6-dien-1-yl)-7-hydroxy-2,2-dimethyl-5-pentyl-4H-benzo[d][1,3]dioxin-4-one

![2-[(2E)-3,7-dimethylocta-2,6-dienyl]-5-pentyl-benzene-1,3-diol](/upload/2023/1/d3368536-f417-4bbc-ad56-9fbbdb8e334a.png)
2-[(2E)-3,7-dimethylocta-2,6-dienyl]-5-pentyl-benzene-1,3-diol
| Conditions | Yield |
|---|---|
|
With water; potassium hydroxide; In 1,4-dioxane; at 120 ℃; for 18h; Sealed tube;
|
64% |

Geraniol

Olivetol
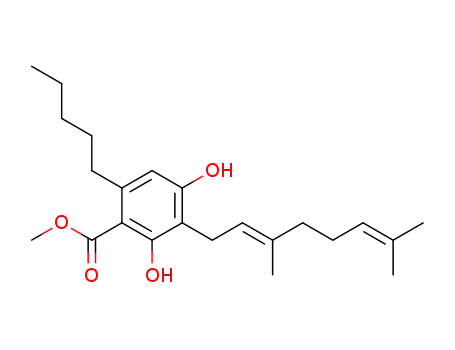
(E)-3-(3,7-dimethylocta-2,6-dien-1-yl)-2,4-dihydroxy-6-pentylbenzoic acid methyl ester
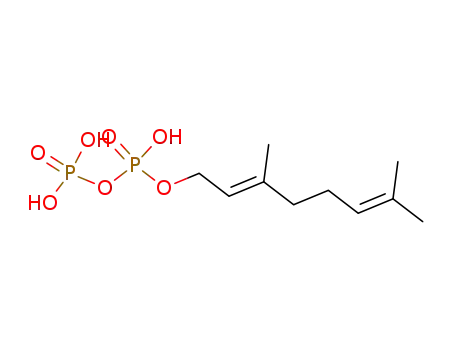
geranyl diphosphate
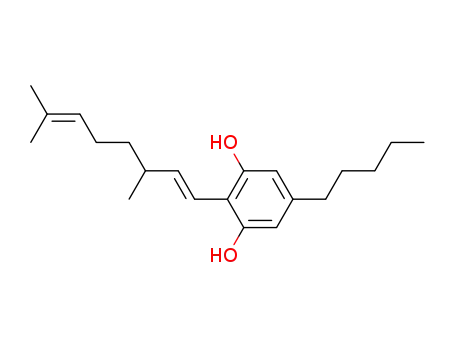
2-(3,7-dimethylocta-1,6-dienyl)-5-pentylresorcinol
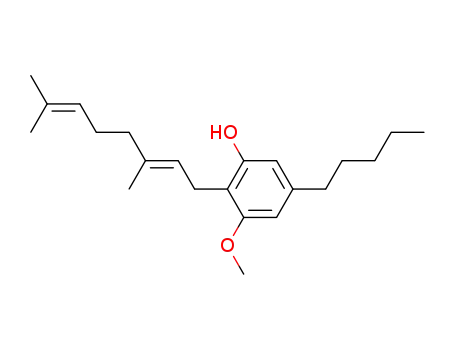
cannabigerol monomethyl ether
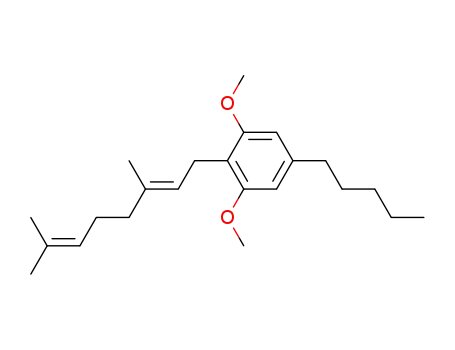
1,3-bis(methoxy)cannabigerol
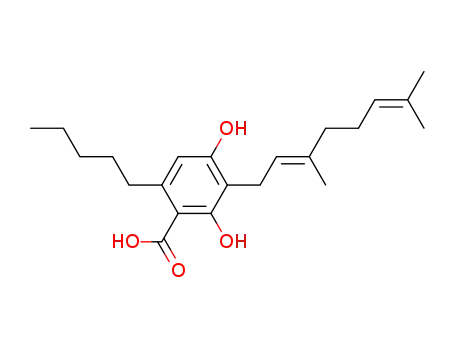
CBGA