Your Location:Home > Products > Cannabis > Cannabigerolic acid

CasNo: 25555-57-1
Molecular Formula: C22H32 O4
|
25555-57-1 Name |
|
|
Name |
Cannabigerolic acid |
|
Synonym |
cannabigerolic acid;2,4-Dihydroxy-3-[(2E)-3,7-dimethyl-2,6-octadienyl]-6-pentylbenzoic acid;3-[(E)-3,7-Dimethyl-2,6-octadienyl]-2,4-dihydroxy-6-pentylbenzoic acid;CBGA;Cannabigerolic acid solution;Cannabigerolic Acid (CRM);(E)-Cannabigerolic Acid;Benzoic acid, 3-[(2E)-3,7-dimethyl-2,6-octadien-1-yl]-2,4-dihydroxy-6-pentyl- |
|
25555-57-1 Chemical & Physical Properties |
|
|
Boiling point |
535.7±50.0 °C at 760 mmHg |
|
Density |
1.1±0.1 g/cm3 |
|
Molecular Formula |
C22H32O4 |
|
Molecular Weight |
360.487 |
|
Flash Point |
291.9±26.6 °C |
|
PSA |
77.76000 |
|
LogP |
8.31 |
|
Exact Mass |
360.230072 |
|
Vapour Pressure |
0.0±1.5 mmHg at 25°C |
|
Index of Refraction |
1.555 |
Cannabigerolic acid (CBGA) is arguably the most important cannabinoid; it is enzymatically converted into other acidic cannabinoids, which subsequently undergo non-enzymatic processes (isomerization, thermal decarboxylation, oxidation, etc.) to synthesize further cannabinoids. Cannabigerolic acid had a binding affinity KD=2.16×10−4 M). Cannabigerolic acid (CBGA), and several other cannabinoids against severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), human immunodeficiency virus (HIV), and γ-herpes viruses.
InChI:InChI=1/C22H32O4/c1-5-6-7-11-17-14-19(23)18(21(24)20(17)22(25)26)13-12-16(4)10-8-9-15(2)3/h9,12,14,23-24H,5-8,10-11,13H2,1-4H3,(H,25,26)/b16-12+
Cannabigerolic acid (CBGA) was the most potent of the cannabinoids studied in preventing hyperthermia-induced seizures in this model. This finding is of particular interest as the dose required indicates that CBGA may actually be more potent at this effect than CBD, although there was no direct comparison in this study.
The cannabinoids are a class of molecules endogenous to the cannabis plant. However, cannabigerolic acid (CBGA) is arguably the most important cannabinoid; it is enzymatically converted into other acidic cannabinoids, which subsequently undergo non-enzymatic processes (isomerization, thermal decarboxylation, oxidation, etc.) to synthesize further cannabinoids.
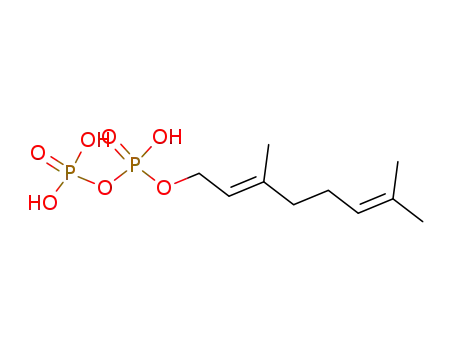
geranyl diphosphate

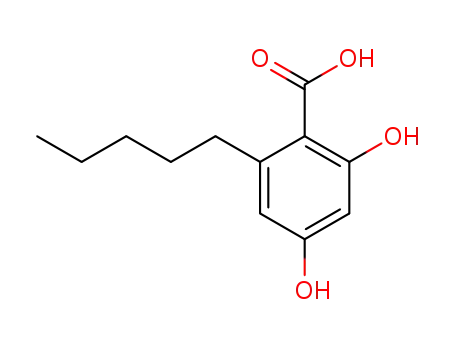
2,4-dihydroxyl-6-pentylbenzoic acid

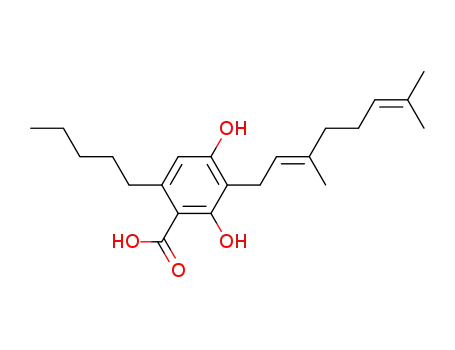
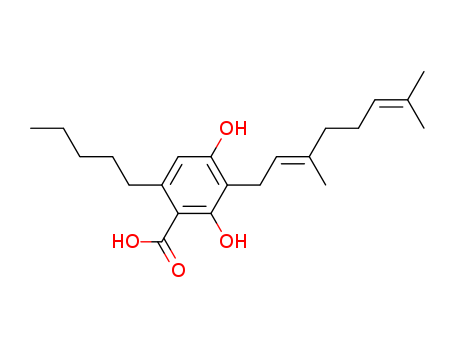
CBGA
| Conditions | Yield |
|---|---|
|
With aromatic prenyltransferase; Enzymatic reaction;
|
![2-[(2E)-3,7-dimethylocta-2,6-dienyl]-5-pentyl-benzene-1,3-diol](/upload/2023/1/17fa0b56-fb6b-4c22-92a5-f7cab83ac362.png)
2-[(2E)-3,7-dimethylocta-2,6-dienyl]-5-pentyl-benzene-1,3-diol

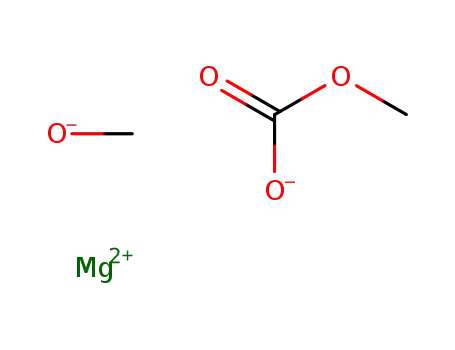
magnesium methyl carbonate


CBGA
| Conditions | Yield |
|---|---|
|
In N,N-dimethyl-formamide; at 120 ℃; for 1h;
|
90% |
|
With carbon dioxide; In N,N-dimethyl-formamide; at 120 ℃; for 2.5h; Time;
|
85% |
|
In N,N-dimethyl-formamide; at 120 ℃; for 1h;
|
|
|
In N,N-dimethyl-formamide; at 120 ℃; for 1h;
|
|
|
In N,N-dimethyl-formamide; at 130 ℃; for 3h;
|
|
|
In N,N-dimethyl-formamide; at 110 ℃; for 2h; Sealed tube; Inert atmosphere;
|
|
|
In N,N-dimethyl-formamide; at 110 ℃; for 2h; Inert atmosphere; Sealed tube;
|
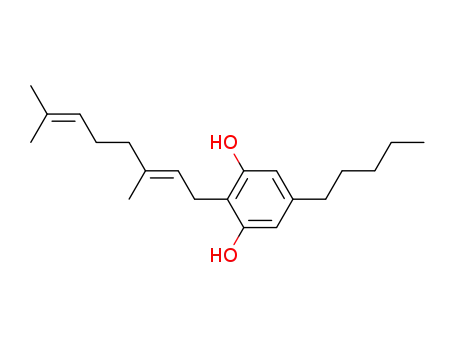
2-[(2E)-3,7-dimethylocta-2,6-dienyl]-5-pentyl-benzene-1,3-diol

magnesium methyl carbonate
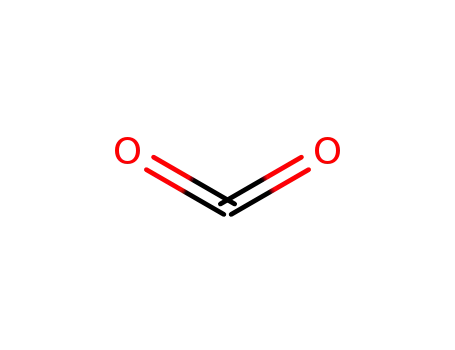
carbon dioxide
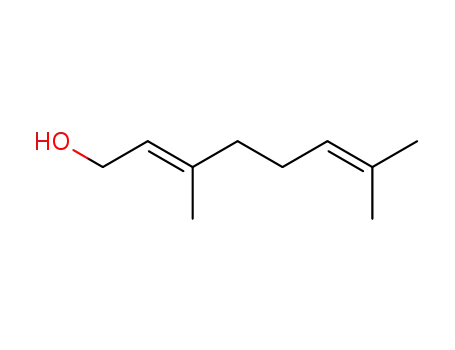
Geraniol
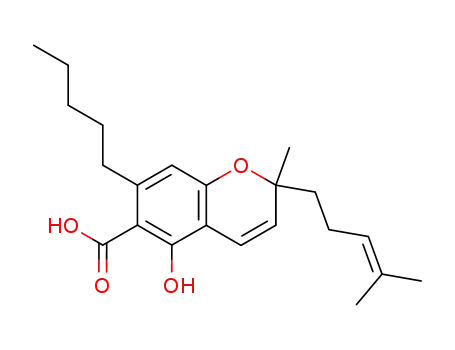
6-carboxy-2-methyl-2-(4-methylpent-3-enyl)-7-pentylchromen-5-ol
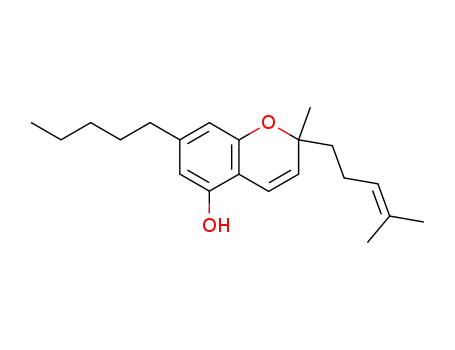
cannabichromene
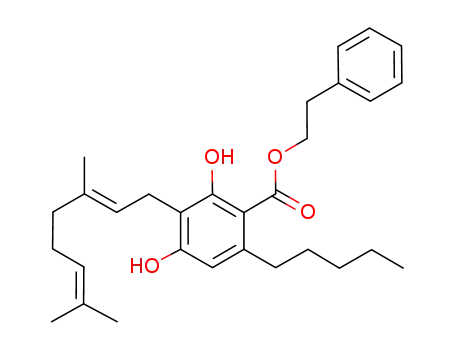
pre-cannabigerol phenethyl ester
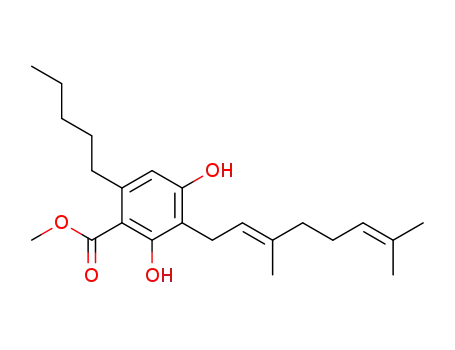
(E)-3-(3,7-dimethylocta-2,6-dien-1-yl)-2,4-dihydroxy-6-pentylbenzoic acid methyl ester