Your Location:Home > Products > Cannabis > 5-butylbenzene-1,3-diol
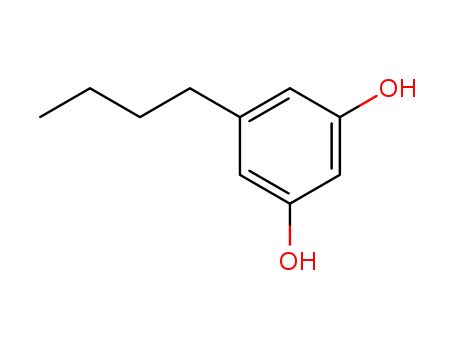

CasNo: 46113-76-2
Molecular Formula: C10H14O2
|
46113-76-2 Name |
|
|
Name |
5-butylbenzene-1,3-diol |
|
Synonym |
5-butylbenzene-1,3-diol;CPDD1006;5-Butyl-1,3-benzenediol;1,3-Benzenediol, 5-butyl- |
|
46113-76-2 Chemical & Physical Properties |
|
|
Melting point |
81.5-82.5 °C |
|
Boiling point |
151-152 °C |
|
Density |
1.092±0.06 g/cm3(Predicted) |
|
Molecular Formula |
C10H14O2 |
|
Molecular Weight |
166.21700 |
|
PSA |
40.46000 |
|
LogP |
2.44040 |
|
Exact Mass |
166.09900 |
The chemical formula of 1,3-Benzenediol, 5-butyl- is C10H14O2 which containing 10 Carbon atoms,14 Hydrogen atoms and 2 Oxygen atoms,and the molecular weight of 1,3-Benzenediol, 5-butyl- is 166.22.
The latter was prepared by Friedel-Craft allylation of 5-butylbenzene-1,3-diol with (1S,4R)-1-methyl-4-(prop-1-en-2-yl)cycloex-2-enol, using pTSA as catalyst as reported in Scheme 1. …One of the major impurities of CBD extracted from hemp, cannabidibutol (CBDB), was fully characterized for the first time. A stereoselective synthesis was developed in order to confirm its identity and stereochemistry.
It was performed initially by direct condensation of 5-butylbenzene-1,3-diol with (1S,4R)-1-… The Friedel-Craft allylation of 5-butylbenzene-1,3-diol with (1S,4R)-1-methyl-4-(prop-1-en-2-…The tetrad test in mice showed a partial agonistic activity of Δ9-THCB toward the CB1 receptor.
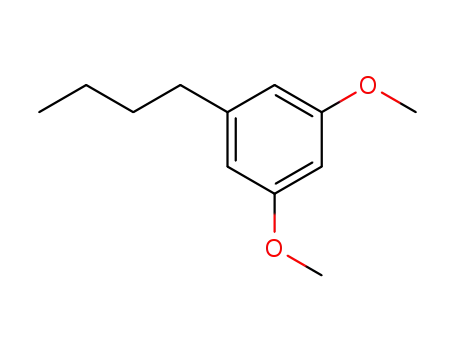
di-methyl ether of 5-n-butyl-resorcinol

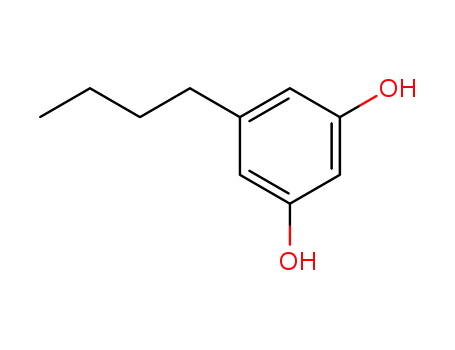
5-n-butyl-1,3-dihydroxybenzene
| Conditions | Yield |
|---|---|
|
With boron tribromide; In dichloromethane; at 0 - 20 ℃;
|
78% |
|
di-methyl ether of 5-n-butyl-resorcinol; With boron tribromide; In dichloromethane; at -15 - 20 ℃; for 2h; Inert atmosphere;
With water; In dichloromethane;
|
75% |
|
With boron tribromide; In dichloromethane; at 0 - 20 ℃; for 20h; Inert atmosphere;
|
65% |
|
With hydrogen iodide;
|
|
|
With chloro-trimethyl-silane; sodium iodide; In acetonitrile; for 36h; Heating;
|
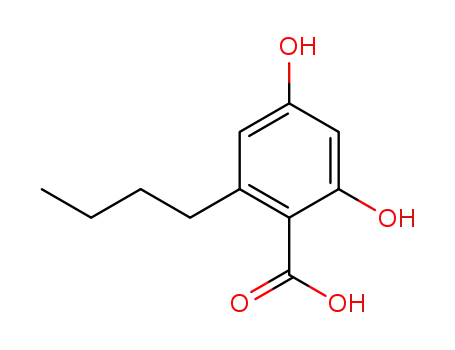
2,4-dihydroxy-6-n-butylbenzoic acid


5-n-butyl-1,3-dihydroxybenzene
| Conditions | Yield |
|---|---|
|
In methanol; water; at 60 ℃; for 5h;
|
80% |

di-methyl ether of 5-n-butyl-resorcinol
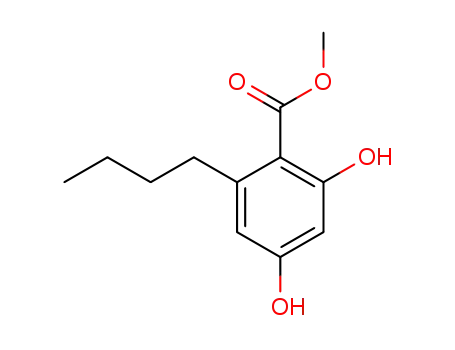
2,4-dihydroxy-6-n-butylbenzoic acid, methyl ester
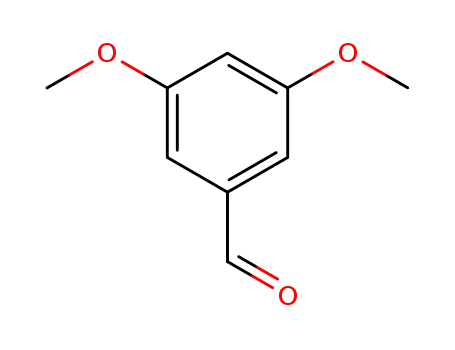
3,5-dimethoxybenzaldehdye
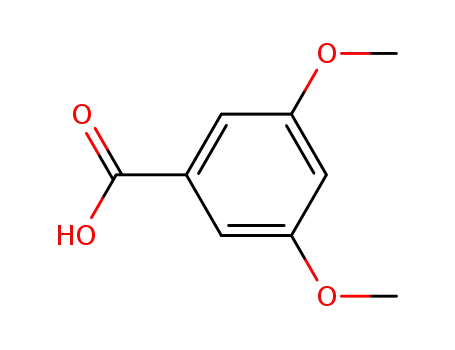
3,5-dimethoxybenzoic acid
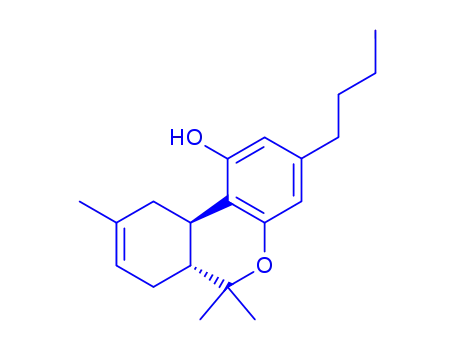
(6aR,10aR)-3-butyl-6,6,9-trimethyl-6a,7,10,10a-tetrahydro-6H-benzo[c]chromen-1-ol
CBD